Zinc »
PDB 5lsz-5m2x »
5lzl »
Zinc in PDB 5lzl: Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase
Enzymatic activity of Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase
All present enzymatic activity of Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase:
4.2.1.24;
4.2.1.24;
Protein crystallography data
The structure of Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase, PDB code: 5lzl
was solved by
N.Azim,
P.T.Erskine,
J.Guo,
J.B.Cooper,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 178.02 / 3.47 |
| Space group | P 31 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 205.561, 205.561, 199.171, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 14.8 / 25 |
Zinc Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 20; Page 3, Binding sites: 21 - 24;Binding sites:
The binding sites of Zinc atom in the Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase (pdb code 5lzl). This binding sites where shown within 5.0 Angstroms radius around Zinc atom.In total 24 binding sites of Zinc where determined in the Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase, PDB code: 5lzl:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Zinc binding site 1 out of 24 in 5lzl
Go back to
Zinc binding site 1 out
of 24 in the Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase
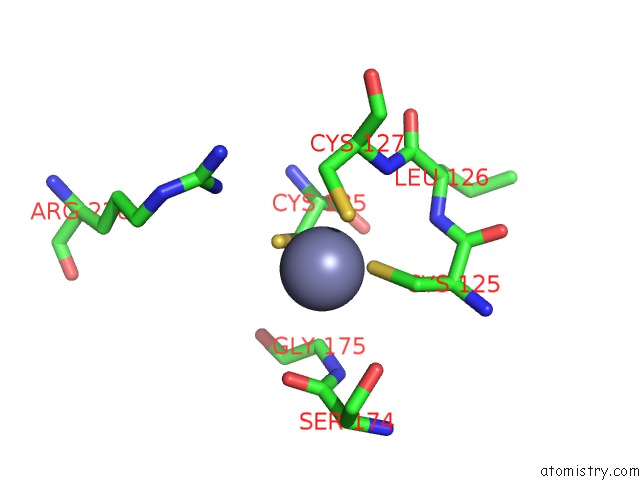
Mono view
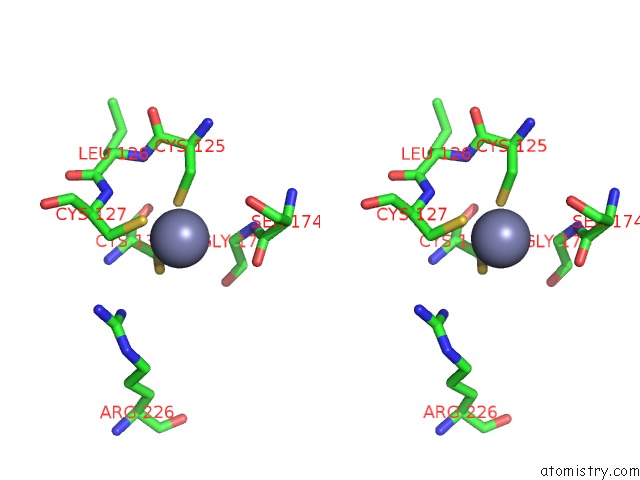
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase within 5.0Å range:
|
Zinc binding site 2 out of 24 in 5lzl
Go back to
Zinc binding site 2 out
of 24 in the Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase
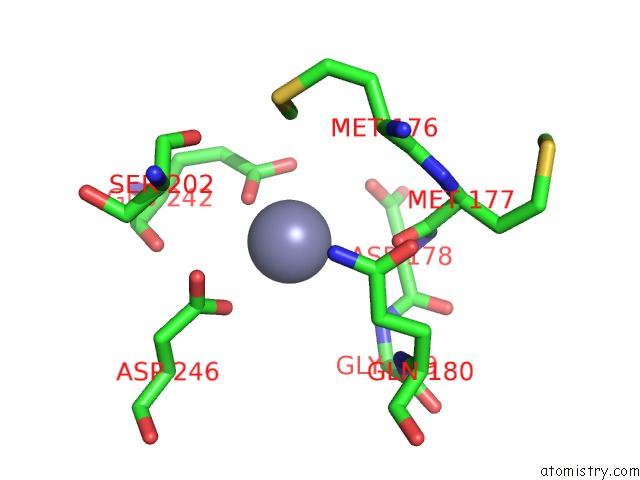
Mono view
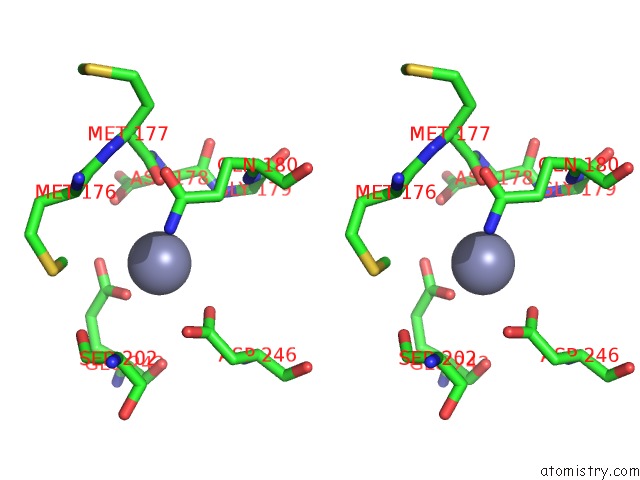
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase within 5.0Å range:
|
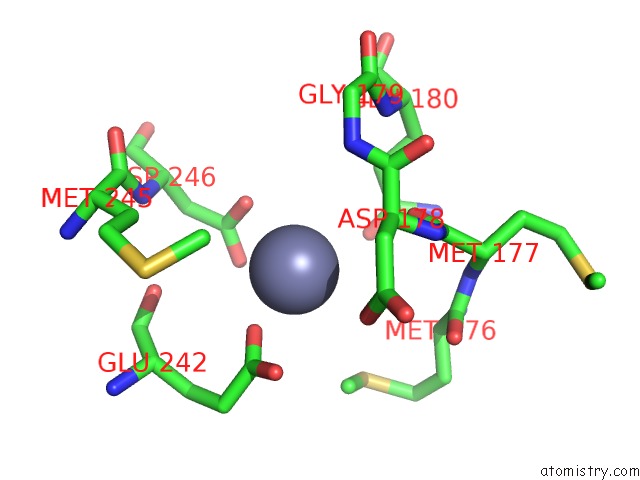
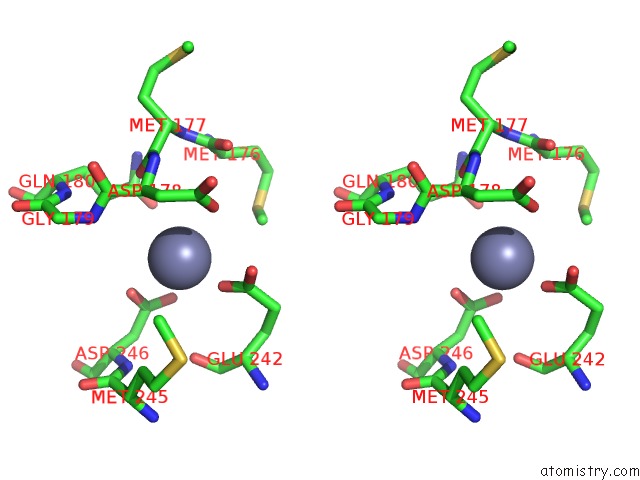
Zinc binding site 3 out of 24 in 5lzl
Go back to
Zinc binding site 3 out
of 24 in the Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase within 5.0Å range:
|
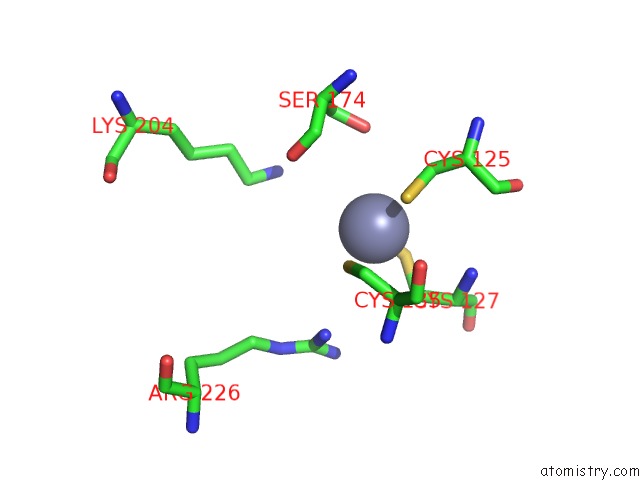
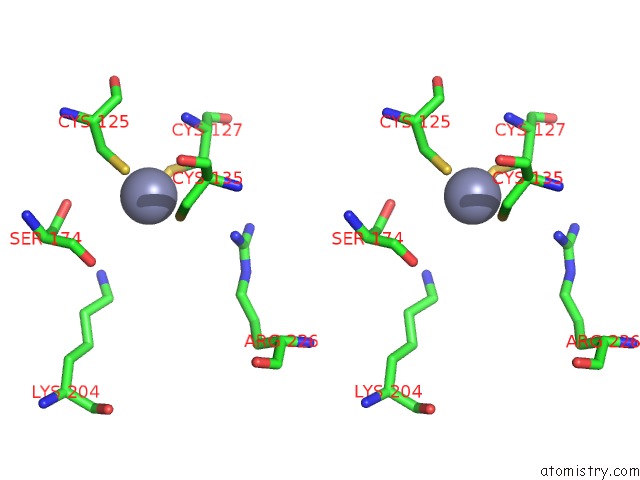
Zinc binding site 4 out of 24 in 5lzl
Go back to
Zinc binding site 4 out
of 24 in the Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase within 5.0Å range:
|
Zinc binding site 5 out of 24 in 5lzl
Go back to
Zinc binding site 5 out
of 24 in the Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase
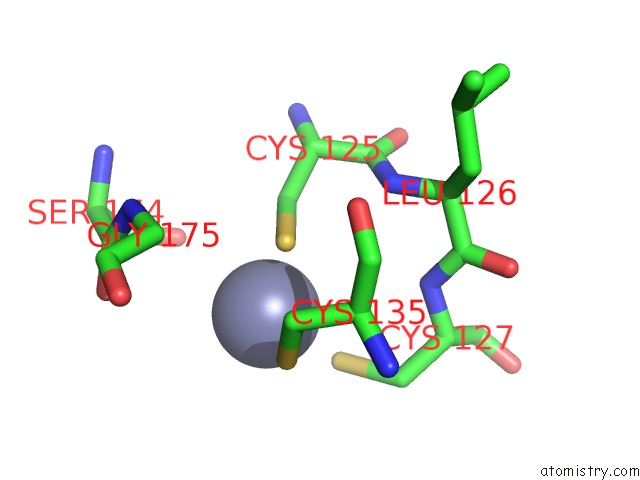
Mono view
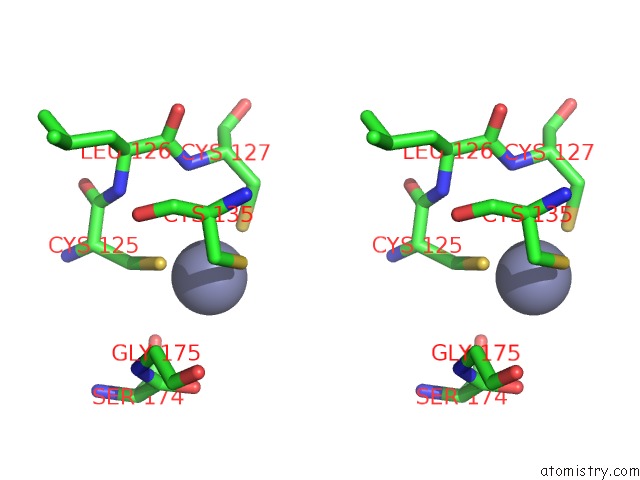
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 5 of Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase within 5.0Å range:
|
Zinc binding site 6 out of 24 in 5lzl
Go back to
Zinc binding site 6 out
of 24 in the Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase
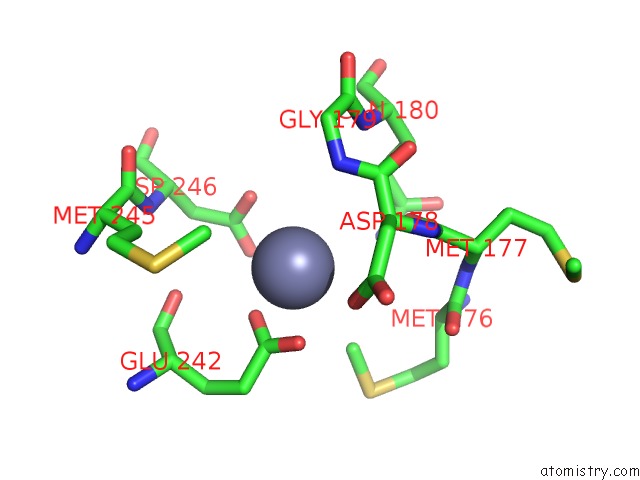
Mono view
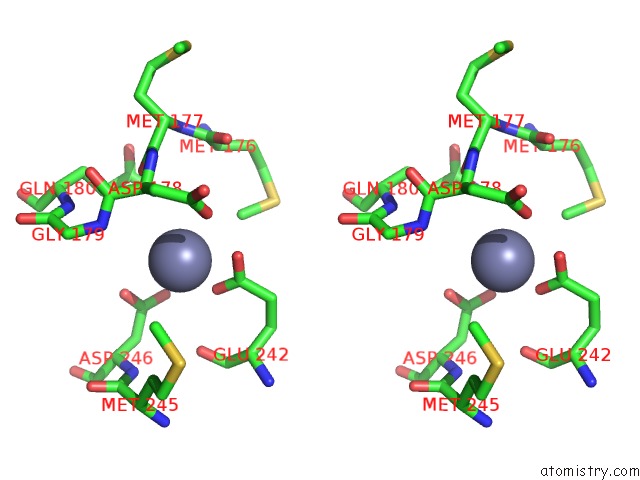
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 6 of Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase within 5.0Å range:
|
Zinc binding site 7 out of 24 in 5lzl
Go back to
Zinc binding site 7 out
of 24 in the Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase
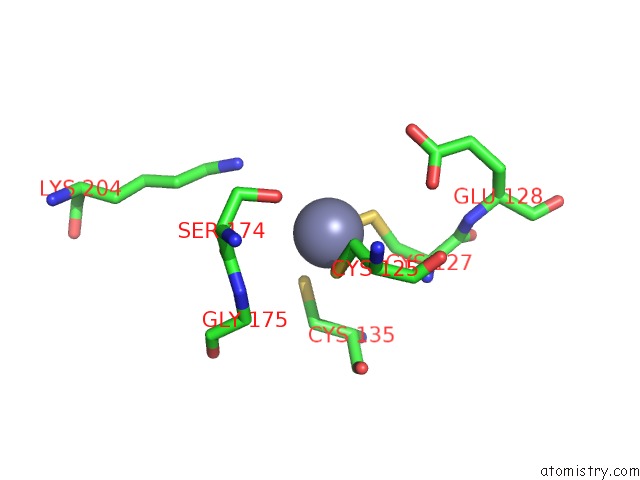
Mono view
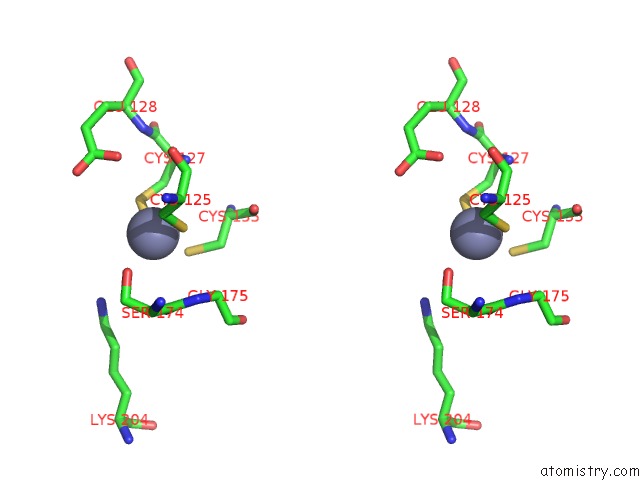
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 7 of Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase within 5.0Å range:
|
Zinc binding site 8 out of 24 in 5lzl
Go back to
Zinc binding site 8 out
of 24 in the Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase
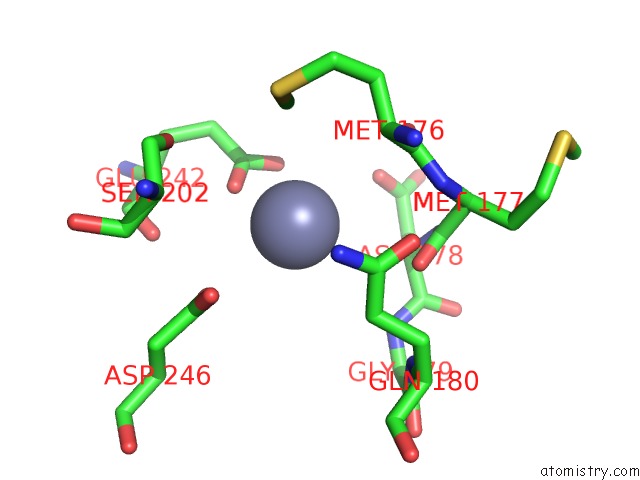
Mono view
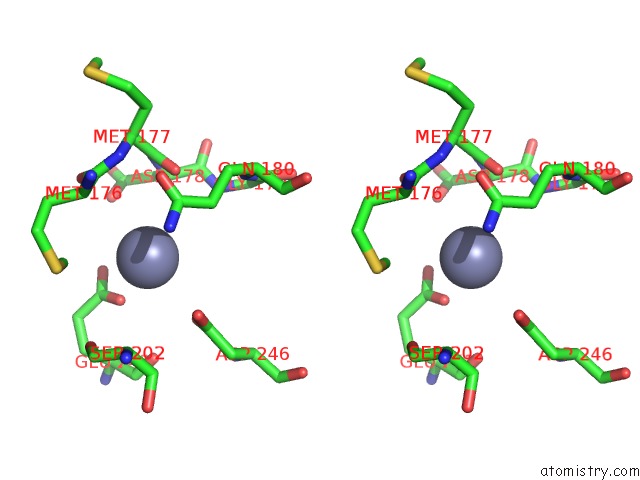
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 8 of Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase within 5.0Å range:
|
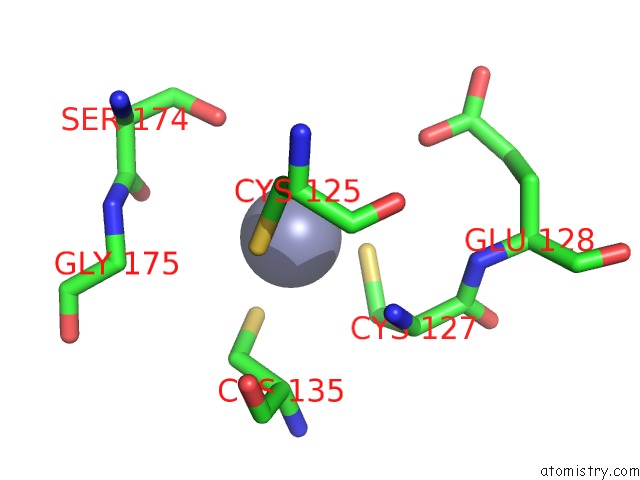
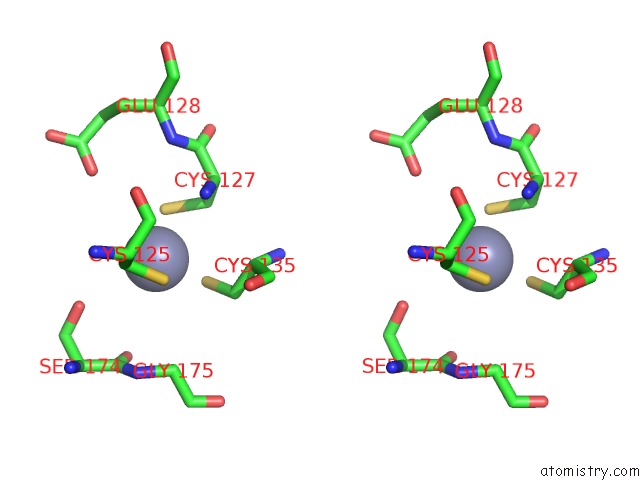
Zinc binding site 9 out of 24 in 5lzl
Go back to
Zinc binding site 9 out
of 24 in the Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 9 of Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase within 5.0Å range:
|
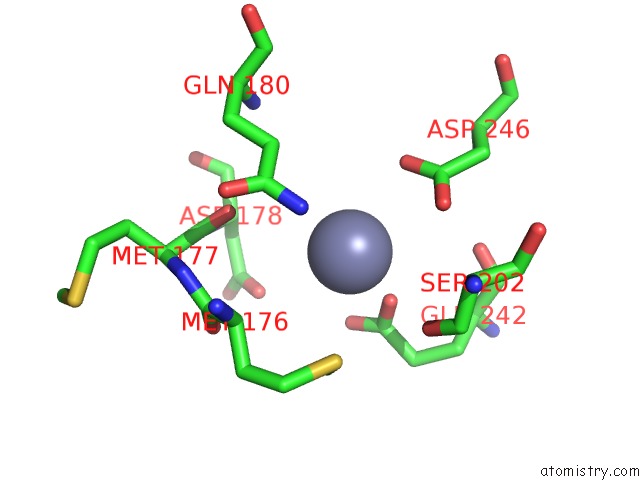
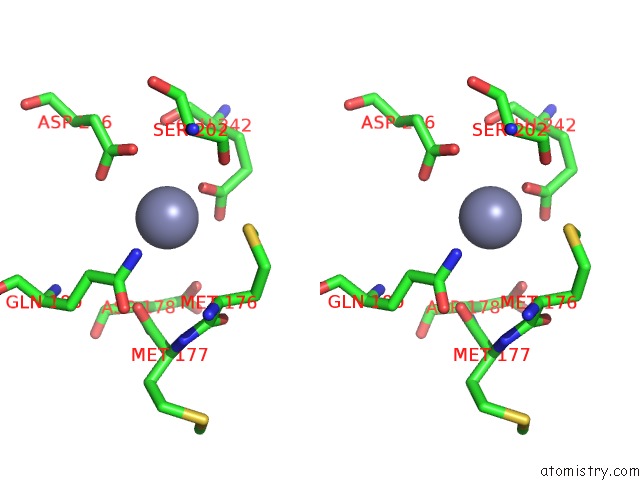
Zinc binding site 10 out of 24 in 5lzl
Go back to
Zinc binding site 10 out
of 24 in the Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 10 of Pyrobaculum Calidifontis 5-Aminolaevulinic Acid Dehydratase within 5.0Å range:
|
Reference:
N.Mills-Davies,
D.Butler,
E.Norton,
D.Thompson,
M.Sarwar,
J.Guo,
R.Gill,
N.Azim,
A.Coker,
S.P.Wood,
P.T.Erskine,
L.Coates,
J.B.Cooper,
N.Rashid,
M.Akhtar,
P.M.Shoolingin-Jordan.
Structural Studies of Substrate and Product Complexes of 5-Aminolaevulinic Acid Dehydratase From Humans, Escherichia Coli and the Hyperthermophile Pyrobaculum Calidifontis. Acta Crystallogr D Struct V. 73 9 2017BIOL.
ISSN: ISSN 2059-7983
PubMed: 28045381
DOI: 10.1107/S2059798316019525
Page generated: Sun Oct 27 21:35:54 2024
ISSN: ISSN 2059-7983
PubMed: 28045381
DOI: 10.1107/S2059798316019525
Last articles
Mn in 1QNMMn in 1QL6
Mn in 1QF3
Mn in 1QLE
Mn in 1QH3
Mn in 1QDO
Mn in 1QGL
Mn in 1QGQ
Mn in 1PQ3
Mn in 1QDC