Zinc »
PDB 5jf2-5jmz »
5jg6 »
Zinc in PDB 5jg6: APC11-Ubv Shows Role of Noncovalent Ring-Ubiquitin Interactions in Processive Multiubiquitination and Ubiquitin Chain Elongation By Apc/C
Protein crystallography data
The structure of APC11-Ubv Shows Role of Noncovalent Ring-Ubiquitin Interactions in Processive Multiubiquitination and Ubiquitin Chain Elongation By Apc/C, PDB code: 5jg6
was solved by
N.G.Brown,
W.Zhang,
S.Yu,
D.J.Miller,
S.S.Sidhu,
B.A.Schulman,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 36.33 / 2.00 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 131.066, 35.193, 72.768, 90.00, 120.42, 90.00 |
| R / Rfree (%) | 18.5 / 22 |
Zinc Binding Sites:
The binding sites of Zinc atom in the APC11-Ubv Shows Role of Noncovalent Ring-Ubiquitin Interactions in Processive Multiubiquitination and Ubiquitin Chain Elongation By Apc/C
(pdb code 5jg6). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 6 binding sites of Zinc where determined in the APC11-Ubv Shows Role of Noncovalent Ring-Ubiquitin Interactions in Processive Multiubiquitination and Ubiquitin Chain Elongation By Apc/C, PDB code: 5jg6:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Zinc where determined in the APC11-Ubv Shows Role of Noncovalent Ring-Ubiquitin Interactions in Processive Multiubiquitination and Ubiquitin Chain Elongation By Apc/C, PDB code: 5jg6:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6;
Zinc binding site 1 out of 6 in 5jg6
Go back to
Zinc binding site 1 out
of 6 in the APC11-Ubv Shows Role of Noncovalent Ring-Ubiquitin Interactions in Processive Multiubiquitination and Ubiquitin Chain Elongation By Apc/C
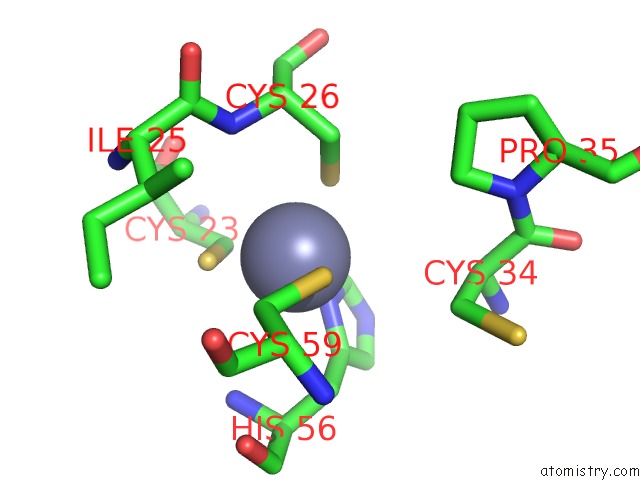
Mono view
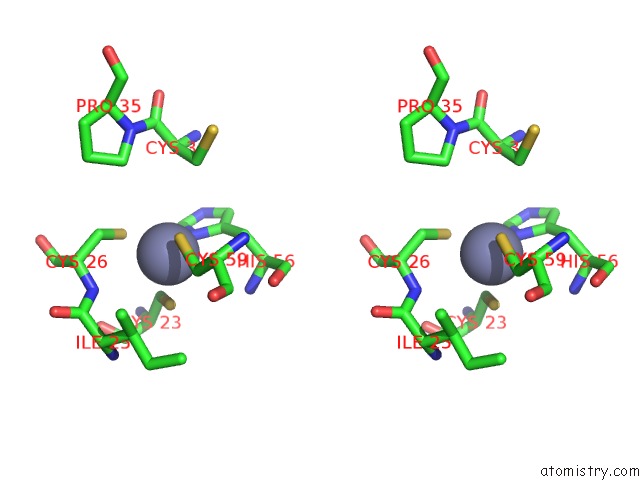
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of APC11-Ubv Shows Role of Noncovalent Ring-Ubiquitin Interactions in Processive Multiubiquitination and Ubiquitin Chain Elongation By Apc/C within 5.0Å range:
|
Zinc binding site 2 out of 6 in 5jg6
Go back to
Zinc binding site 2 out
of 6 in the APC11-Ubv Shows Role of Noncovalent Ring-Ubiquitin Interactions in Processive Multiubiquitination and Ubiquitin Chain Elongation By Apc/C
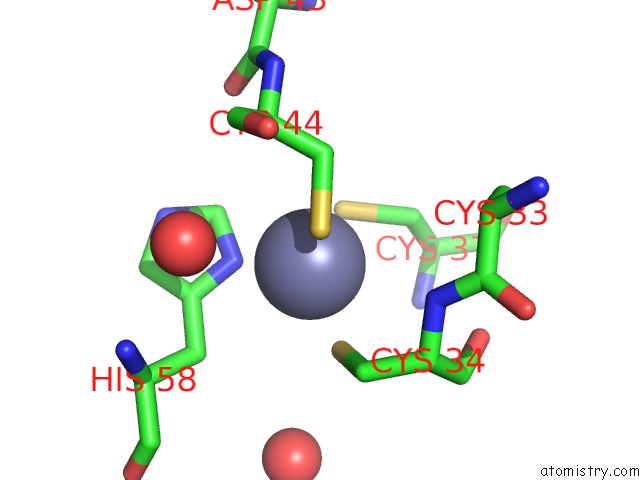
Mono view
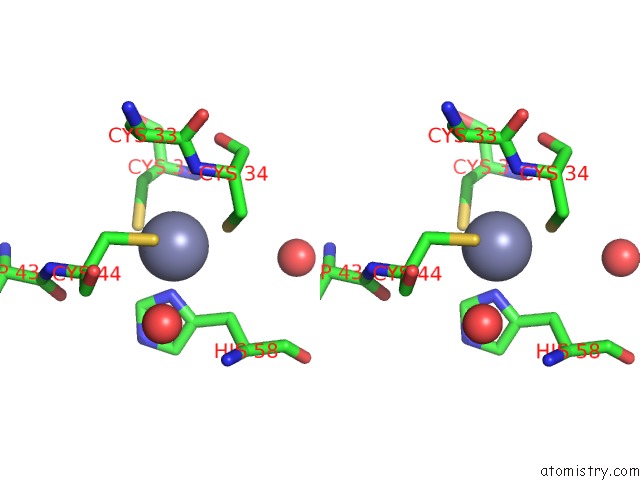
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of APC11-Ubv Shows Role of Noncovalent Ring-Ubiquitin Interactions in Processive Multiubiquitination and Ubiquitin Chain Elongation By Apc/C within 5.0Å range:
|
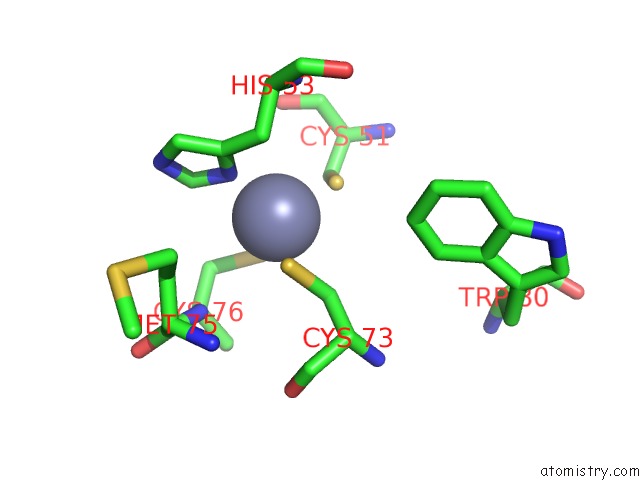
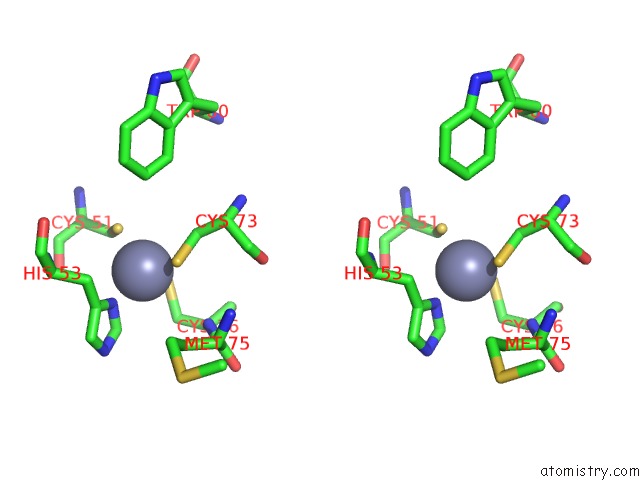
Zinc binding site 3 out of 6 in 5jg6
Go back to
Zinc binding site 3 out
of 6 in the APC11-Ubv Shows Role of Noncovalent Ring-Ubiquitin Interactions in Processive Multiubiquitination and Ubiquitin Chain Elongation By Apc/C
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of APC11-Ubv Shows Role of Noncovalent Ring-Ubiquitin Interactions in Processive Multiubiquitination and Ubiquitin Chain Elongation By Apc/C within 5.0Å range:
|
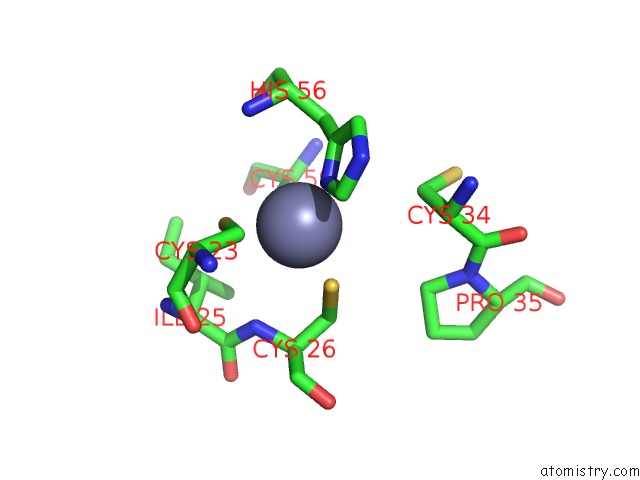
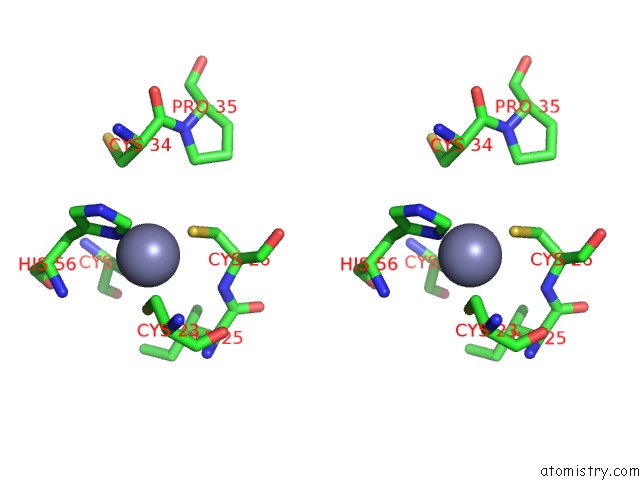
Zinc binding site 4 out of 6 in 5jg6
Go back to
Zinc binding site 4 out
of 6 in the APC11-Ubv Shows Role of Noncovalent Ring-Ubiquitin Interactions in Processive Multiubiquitination and Ubiquitin Chain Elongation By Apc/C
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of APC11-Ubv Shows Role of Noncovalent Ring-Ubiquitin Interactions in Processive Multiubiquitination and Ubiquitin Chain Elongation By Apc/C within 5.0Å range:
|
Zinc binding site 5 out of 6 in 5jg6
Go back to
Zinc binding site 5 out
of 6 in the APC11-Ubv Shows Role of Noncovalent Ring-Ubiquitin Interactions in Processive Multiubiquitination and Ubiquitin Chain Elongation By Apc/C
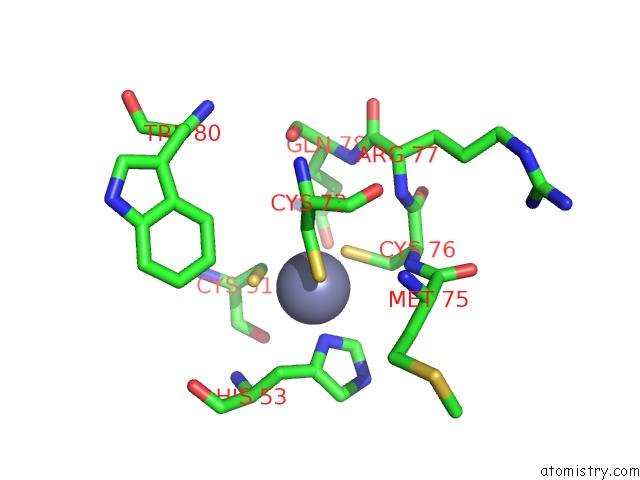
Mono view
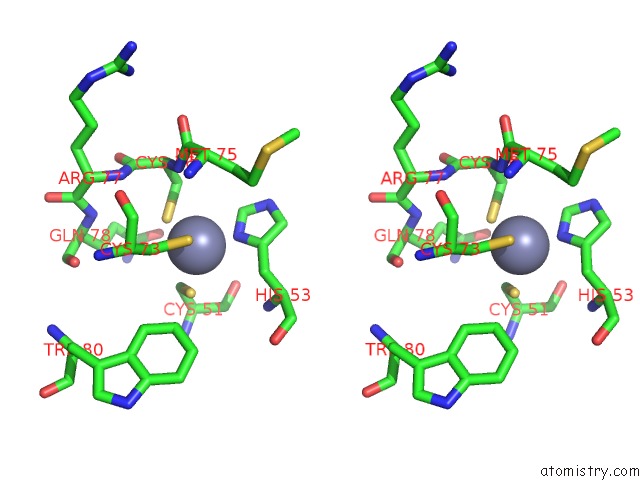
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 5 of APC11-Ubv Shows Role of Noncovalent Ring-Ubiquitin Interactions in Processive Multiubiquitination and Ubiquitin Chain Elongation By Apc/C within 5.0Å range:
|
Zinc binding site 6 out of 6 in 5jg6
Go back to
Zinc binding site 6 out
of 6 in the APC11-Ubv Shows Role of Noncovalent Ring-Ubiquitin Interactions in Processive Multiubiquitination and Ubiquitin Chain Elongation By Apc/C
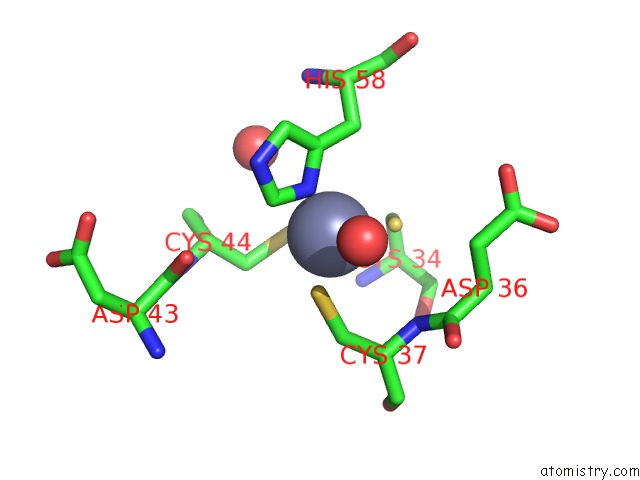
Mono view
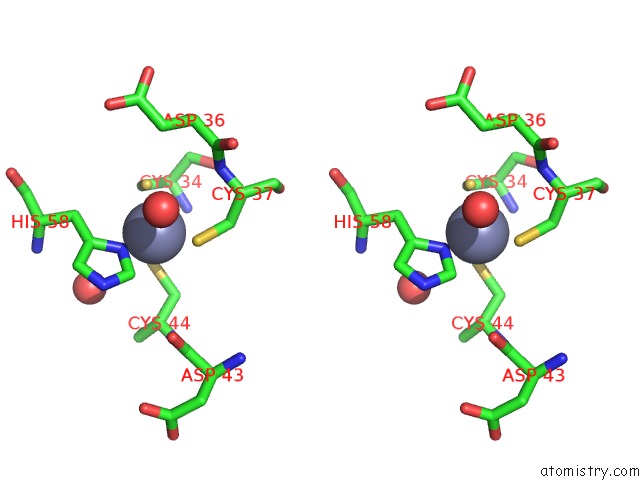
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 6 of APC11-Ubv Shows Role of Noncovalent Ring-Ubiquitin Interactions in Processive Multiubiquitination and Ubiquitin Chain Elongation By Apc/C within 5.0Å range:
|
Reference:
N.G.Brown,
R.Vanderlinden,
E.R.Watson,
F.Weissmann,
A.Ordureau,
K.P.Wu,
W.Zhang,
S.Yu,
P.Y.Mercredi,
J.S.Harrison,
I.F.Davidson,
R.Qiao,
Y.Lu,
P.Dube,
M.R.Brunner,
C.R.Grace,
D.J.Miller,
D.Haselbach,
M.A.Jarvis,
M.Yamaguchi,
D.Yanishevski,
G.Petzold,
S.S.Sidhu,
B.Kuhlman,
M.W.Kirschner,
J.W.Harper,
J.M.Peters,
H.Stark,
B.A.Schulman.
Dual Ring E3 Architectures Regulate Multiubiquitination and Ubiquitin Chain Elongation By Apc/C. Cell V. 165 1440 2016.
ISSN: ISSN 1097-4172
PubMed: 27259151
DOI: 10.1016/J.CELL.2016.05.037
Page generated: Sun Oct 27 18:55:10 2024
ISSN: ISSN 1097-4172
PubMed: 27259151
DOI: 10.1016/J.CELL.2016.05.037
Last articles
Mg in 3LOOMg in 3LMK
Mg in 3LOP
Mg in 3LMI
Mg in 3LOA
Mg in 3LOJ
Mg in 3LM8
Mg in 3LMG
Mg in 3LKM
Mg in 3LD0