Zinc »
PDB 5cdt-5cvm »
5cnx »
Zinc in PDB 5cnx: Crystal Structure of Xaa-Pro Aminopeptidase From Escherichia Coli K12
Protein crystallography data
The structure of Crystal Structure of Xaa-Pro Aminopeptidase From Escherichia Coli K12, PDB code: 5cnx
was solved by
A.Kumar,
V.Are,
B.Ghosh,
S.Jamdar,
R.D.Makde,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.90 / 2.60 |
| Space group | P 32 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 224.202, 224.202, 74.636, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 21.9 / 24 |
Other elements in 5cnx:
The structure of Crystal Structure of Xaa-Pro Aminopeptidase From Escherichia Coli K12 also contains other interesting chemical elements:
| Arsenic | (As) | 3 atoms |
| Sodium | (Na) | 1 atom |
Zinc Binding Sites:
The binding sites of Zinc atom in the Crystal Structure of Xaa-Pro Aminopeptidase From Escherichia Coli K12
(pdb code 5cnx). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 6 binding sites of Zinc where determined in the Crystal Structure of Xaa-Pro Aminopeptidase From Escherichia Coli K12, PDB code: 5cnx:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Zinc where determined in the Crystal Structure of Xaa-Pro Aminopeptidase From Escherichia Coli K12, PDB code: 5cnx:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6;
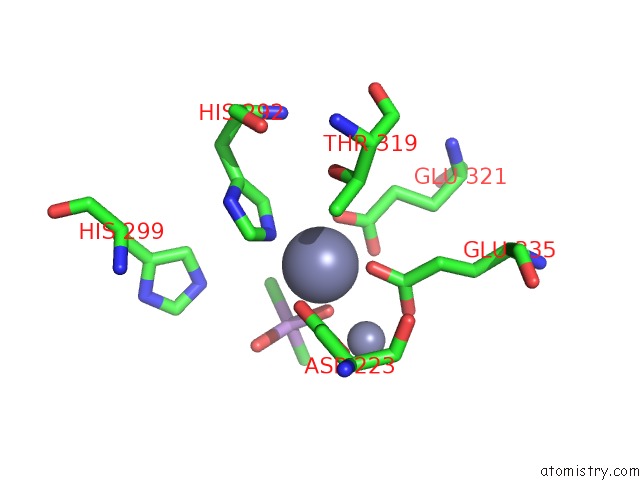
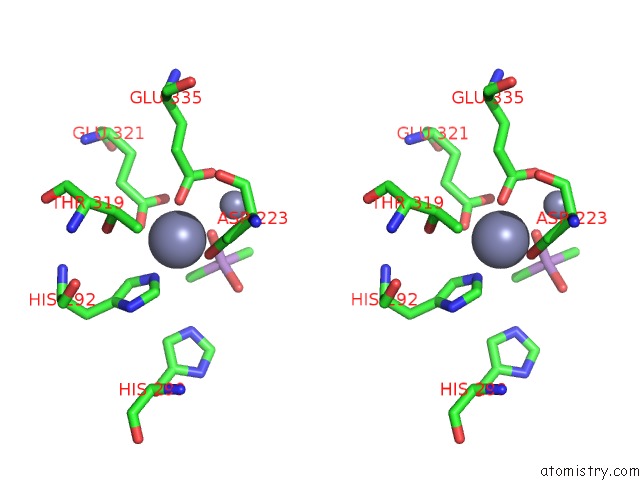
Zinc binding site 1 out of 6 in 5cnx
Go back to
Zinc binding site 1 out
of 6 in the Crystal Structure of Xaa-Pro Aminopeptidase From Escherichia Coli K12
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Crystal Structure of Xaa-Pro Aminopeptidase From Escherichia Coli K12 within 5.0Å range:
|
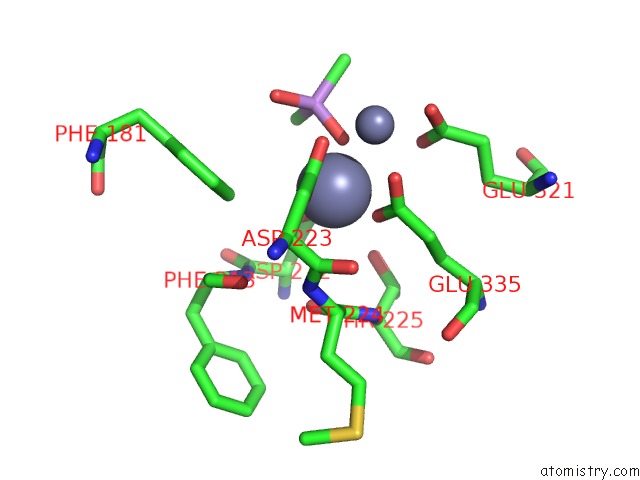
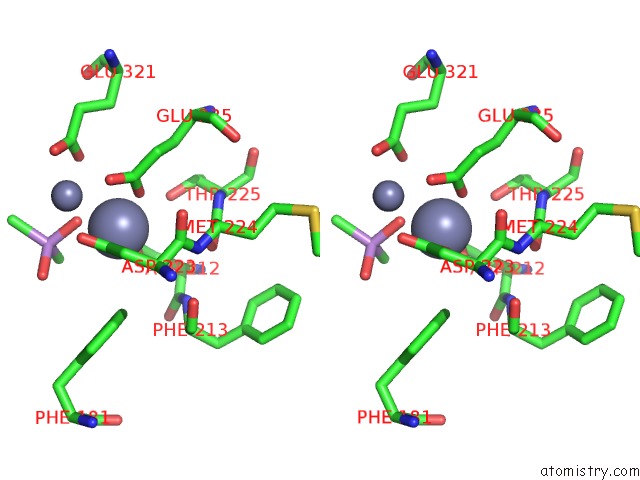
Zinc binding site 2 out of 6 in 5cnx
Go back to
Zinc binding site 2 out
of 6 in the Crystal Structure of Xaa-Pro Aminopeptidase From Escherichia Coli K12
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Crystal Structure of Xaa-Pro Aminopeptidase From Escherichia Coli K12 within 5.0Å range:
|
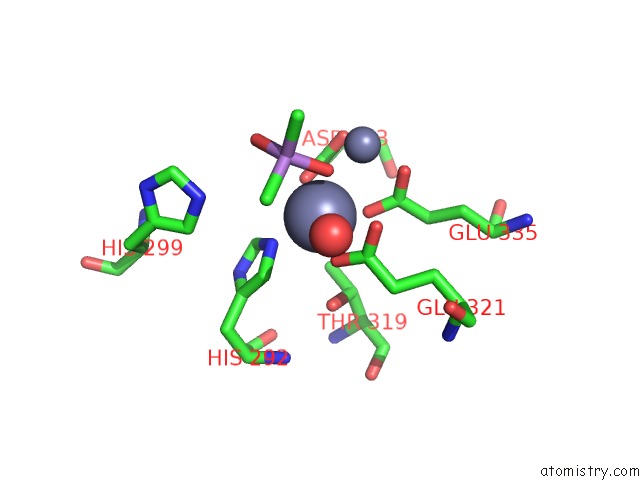
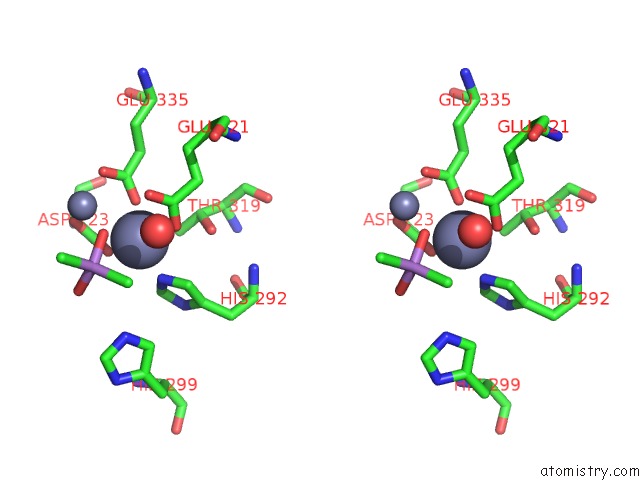
Zinc binding site 3 out of 6 in 5cnx
Go back to
Zinc binding site 3 out
of 6 in the Crystal Structure of Xaa-Pro Aminopeptidase From Escherichia Coli K12
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Crystal Structure of Xaa-Pro Aminopeptidase From Escherichia Coli K12 within 5.0Å range:
|
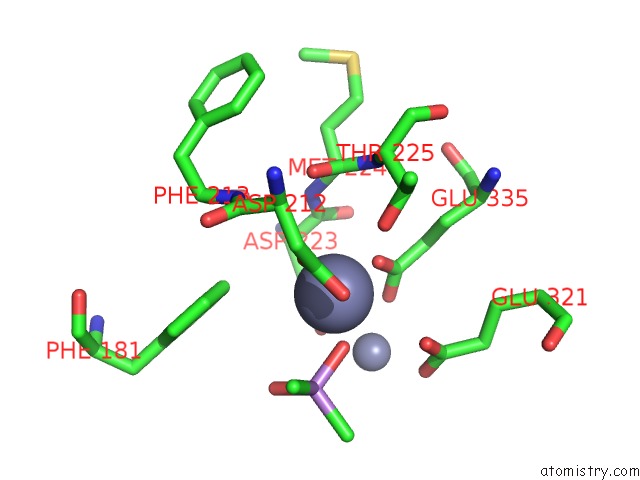
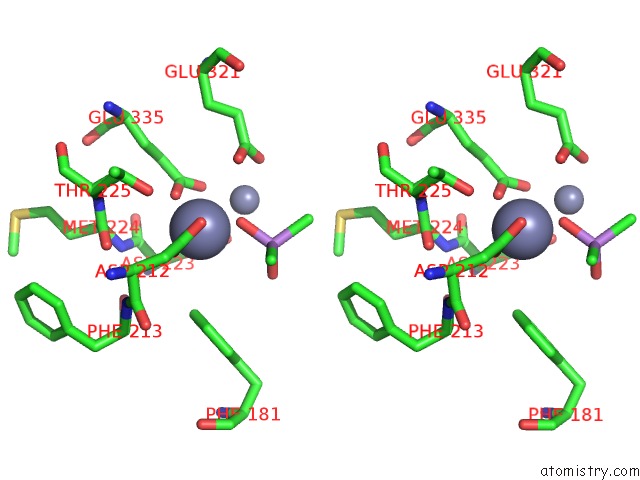
Zinc binding site 4 out of 6 in 5cnx
Go back to
Zinc binding site 4 out
of 6 in the Crystal Structure of Xaa-Pro Aminopeptidase From Escherichia Coli K12
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Crystal Structure of Xaa-Pro Aminopeptidase From Escherichia Coli K12 within 5.0Å range:
|
Zinc binding site 5 out of 6 in 5cnx
Go back to
Zinc binding site 5 out
of 6 in the Crystal Structure of Xaa-Pro Aminopeptidase From Escherichia Coli K12
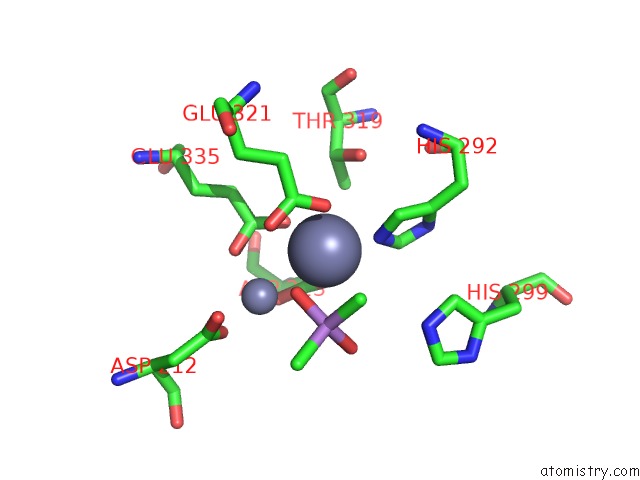
Mono view
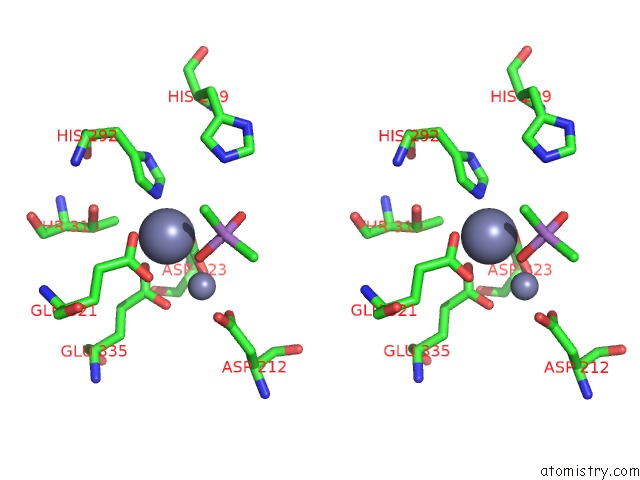
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 5 of Crystal Structure of Xaa-Pro Aminopeptidase From Escherichia Coli K12 within 5.0Å range:
|
Zinc binding site 6 out of 6 in 5cnx
Go back to
Zinc binding site 6 out
of 6 in the Crystal Structure of Xaa-Pro Aminopeptidase From Escherichia Coli K12
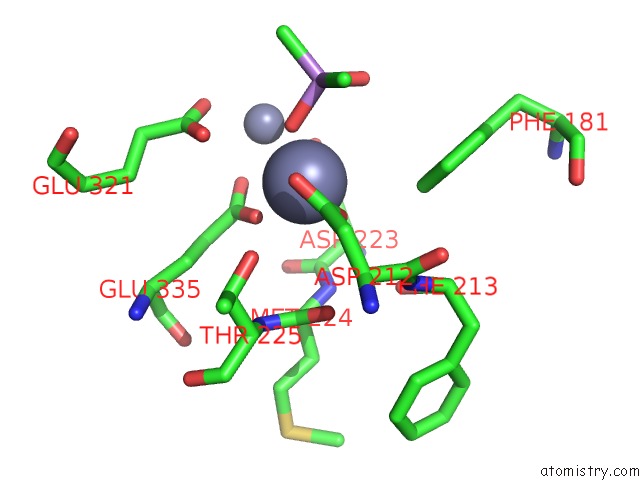
Mono view
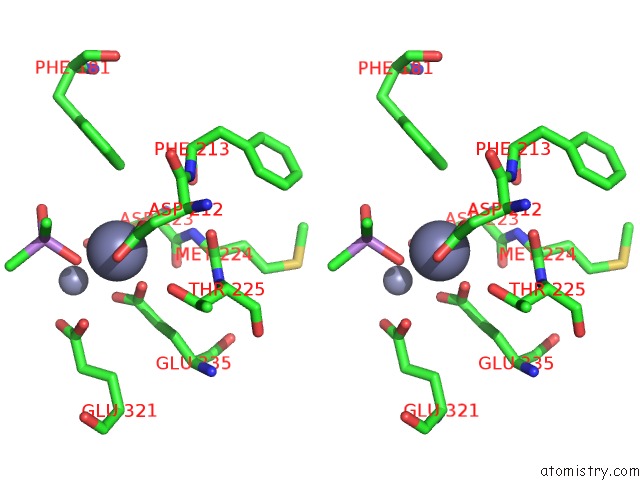
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 6 of Crystal Structure of Xaa-Pro Aminopeptidase From Escherichia Coli K12 within 5.0Å range:
|
Reference:
V.N.Are,
A.Kumar,
V.D.Goyal,
S.S.Gotad,
B.Ghosh,
R.Gadre,
S.N.Jamdar,
R.D.Makde.
Structures and Activities of Widely Conserved Small Prokaryotic Aminopeptidases-P Clarify Classification of M24B Peptidases Proteins 2018.
ISSN: ESSN 1097-0134
PubMed: 30536999
DOI: 10.1002/PROT.25641
Page generated: Sun Oct 27 14:24:52 2024
ISSN: ESSN 1097-0134
PubMed: 30536999
DOI: 10.1002/PROT.25641
Last articles
I in 3QD5I in 3QF1
I in 3QH9
I in 3QGN
I in 3Q7S
I in 3Q5M
I in 3Q1E
I in 3PMC
I in 3PM6
I in 3PY4