Zinc »
PDB 3cjp-3czs »
3csq »
Zinc in PDB 3csq: Crystal and Cryoem Structural Studies of A Cell Wall Degrading Enzyme in the Bacteriophage PHI29 Tail
Protein crystallography data
The structure of Crystal and Cryoem Structural Studies of A Cell Wall Degrading Enzyme in the Bacteriophage PHI29 Tail, PDB code: 3csq
was solved by
Y.Xiang,
M.G.Rossmann,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.90 / 1.80 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 53.842, 133.963, 85.725, 90.00, 89.99, 90.00 |
| R / Rfree (%) | 23.8 / 28.2 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Crystal and Cryoem Structural Studies of A Cell Wall Degrading Enzyme in the Bacteriophage PHI29 Tail
(pdb code 3csq). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 4 binding sites of Zinc where determined in the Crystal and Cryoem Structural Studies of A Cell Wall Degrading Enzyme in the Bacteriophage PHI29 Tail, PDB code: 3csq:
Jump to Zinc binding site number: 1; 2; 3; 4;
In total 4 binding sites of Zinc where determined in the Crystal and Cryoem Structural Studies of A Cell Wall Degrading Enzyme in the Bacteriophage PHI29 Tail, PDB code: 3csq:
Jump to Zinc binding site number: 1; 2; 3; 4;
Zinc binding site 1 out of 4 in 3csq
Go back to
Zinc binding site 1 out
of 4 in the Crystal and Cryoem Structural Studies of A Cell Wall Degrading Enzyme in the Bacteriophage PHI29 Tail
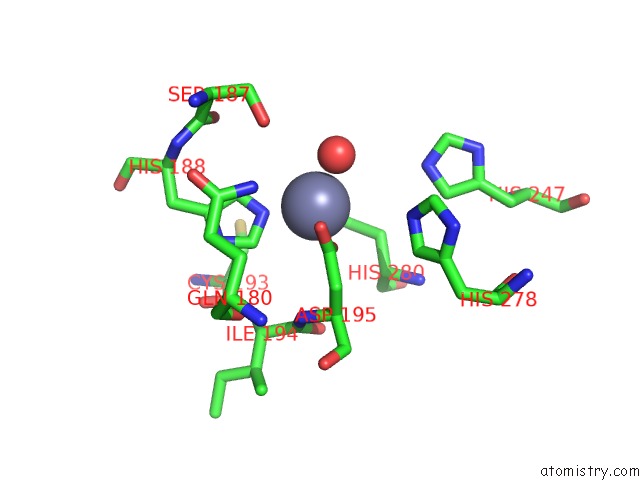
Mono view
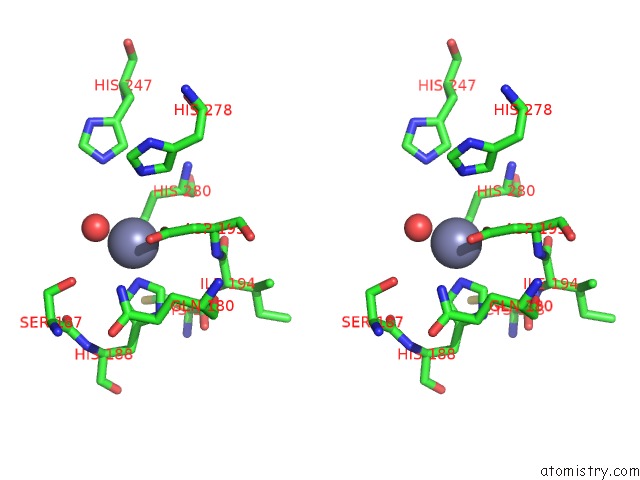
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Crystal and Cryoem Structural Studies of A Cell Wall Degrading Enzyme in the Bacteriophage PHI29 Tail within 5.0Å range:
|
Zinc binding site 2 out of 4 in 3csq
Go back to
Zinc binding site 2 out
of 4 in the Crystal and Cryoem Structural Studies of A Cell Wall Degrading Enzyme in the Bacteriophage PHI29 Tail
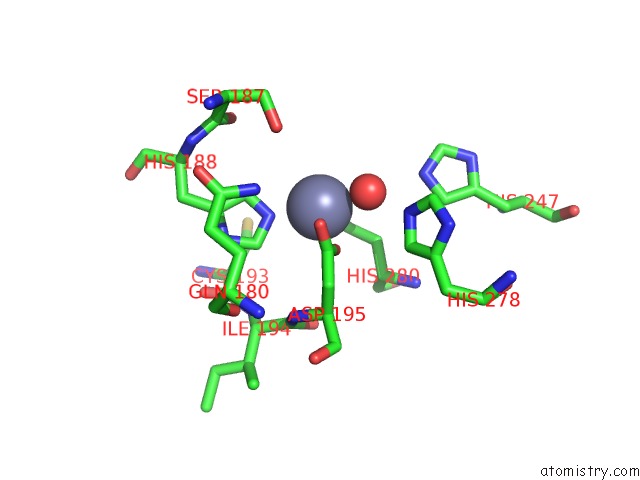
Mono view
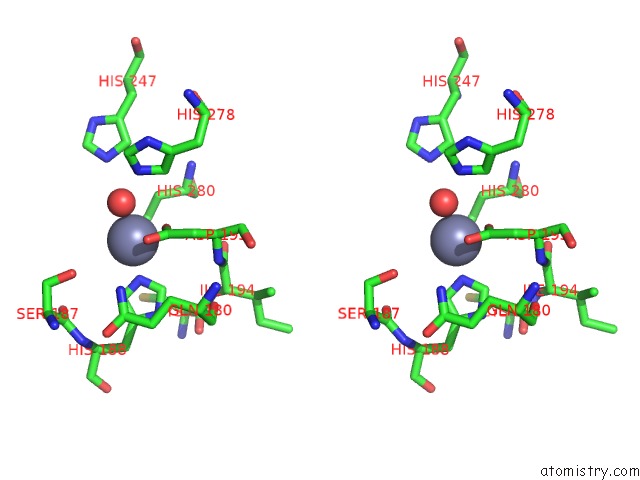
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Crystal and Cryoem Structural Studies of A Cell Wall Degrading Enzyme in the Bacteriophage PHI29 Tail within 5.0Å range:
|
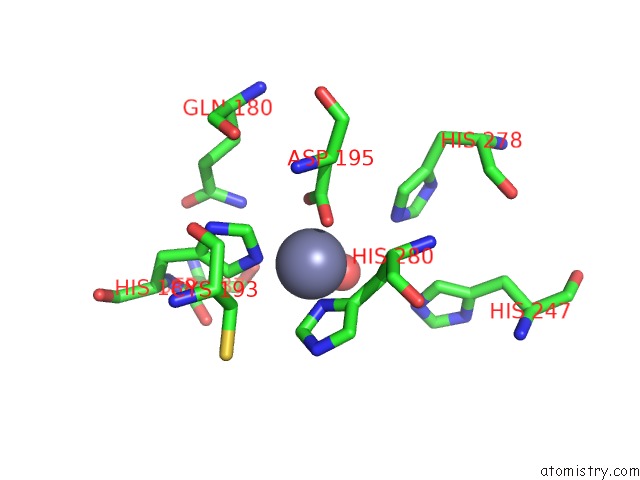
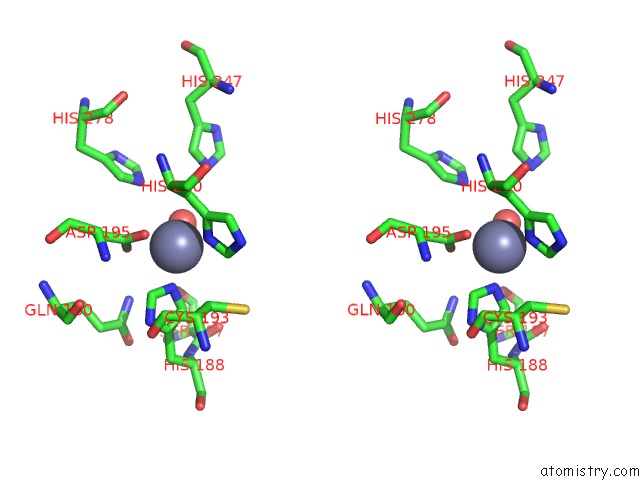
Zinc binding site 3 out of 4 in 3csq
Go back to
Zinc binding site 3 out
of 4 in the Crystal and Cryoem Structural Studies of A Cell Wall Degrading Enzyme in the Bacteriophage PHI29 Tail
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Crystal and Cryoem Structural Studies of A Cell Wall Degrading Enzyme in the Bacteriophage PHI29 Tail within 5.0Å range:
|
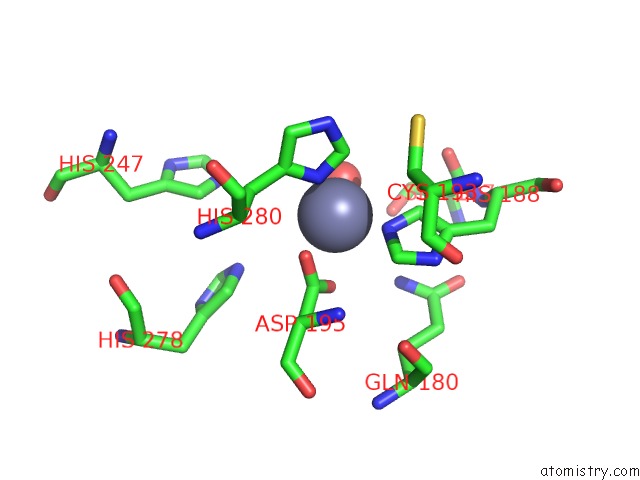
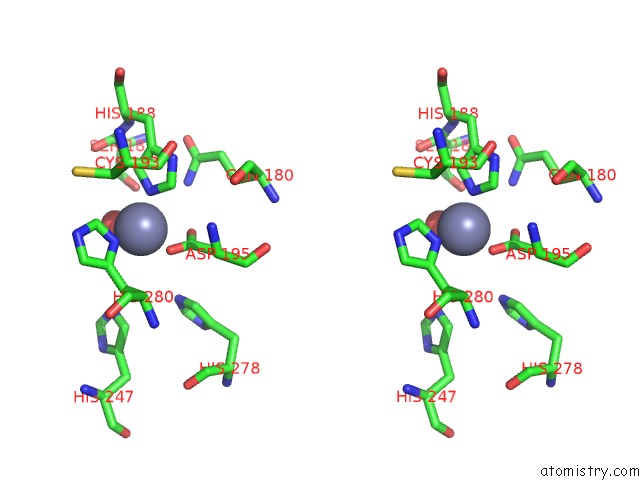
Zinc binding site 4 out of 4 in 3csq
Go back to
Zinc binding site 4 out
of 4 in the Crystal and Cryoem Structural Studies of A Cell Wall Degrading Enzyme in the Bacteriophage PHI29 Tail
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Crystal and Cryoem Structural Studies of A Cell Wall Degrading Enzyme in the Bacteriophage PHI29 Tail within 5.0Å range:
|
Reference:
Y.Xiang,
M.C.Morais,
D.N.Cohen,
V.D.Bowman,
D.L.Anderson,
M.G.Rossmann.
Crystal and Cryoem Structural Studies of A Cell Wall Degrading Enzyme in the Bacteriophage PHI29 Tail. Proc.Natl.Acad.Sci.Usa V. 105 9552 2008.
ISSN: ISSN 0027-8424
PubMed: 18606992
DOI: 10.1073/PNAS.0803787105
Page generated: Thu Oct 24 11:56:39 2024
ISSN: ISSN 0027-8424
PubMed: 18606992
DOI: 10.1073/PNAS.0803787105
Last articles
K in 7BT2K in 7BMZ
K in 7BMU
K in 7BH2
K in 7BIL
K in 7BI1
K in 7BH1
K in 7B2H
K in 7B1S
K in 7B80