Zinc »
PDB 2q38-2qm1 »
2qds »
Zinc in PDB 2qds: Crystal Structure of the Zinc Carbapenemase Cpha in Complex with the Inhibitor D-Captopril
Enzymatic activity of Crystal Structure of the Zinc Carbapenemase Cpha in Complex with the Inhibitor D-Captopril
All present enzymatic activity of Crystal Structure of the Zinc Carbapenemase Cpha in Complex with the Inhibitor D-Captopril:
3.5.2.6;
3.5.2.6;
Protein crystallography data
The structure of Crystal Structure of the Zinc Carbapenemase Cpha in Complex with the Inhibitor D-Captopril, PDB code: 2qds
was solved by
G.Garau,
O.Dideberg,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 50.00 / 1.66 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 43.001, 100.990, 116.819, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 14.8 / 17 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Crystal Structure of the Zinc Carbapenemase Cpha in Complex with the Inhibitor D-Captopril
(pdb code 2qds). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total only one binding site of Zinc was determined in the Crystal Structure of the Zinc Carbapenemase Cpha in Complex with the Inhibitor D-Captopril, PDB code: 2qds:
In total only one binding site of Zinc was determined in the Crystal Structure of the Zinc Carbapenemase Cpha in Complex with the Inhibitor D-Captopril, PDB code: 2qds:
Zinc binding site 1 out of 1 in 2qds
Go back to
Zinc binding site 1 out
of 1 in the Crystal Structure of the Zinc Carbapenemase Cpha in Complex with the Inhibitor D-Captopril
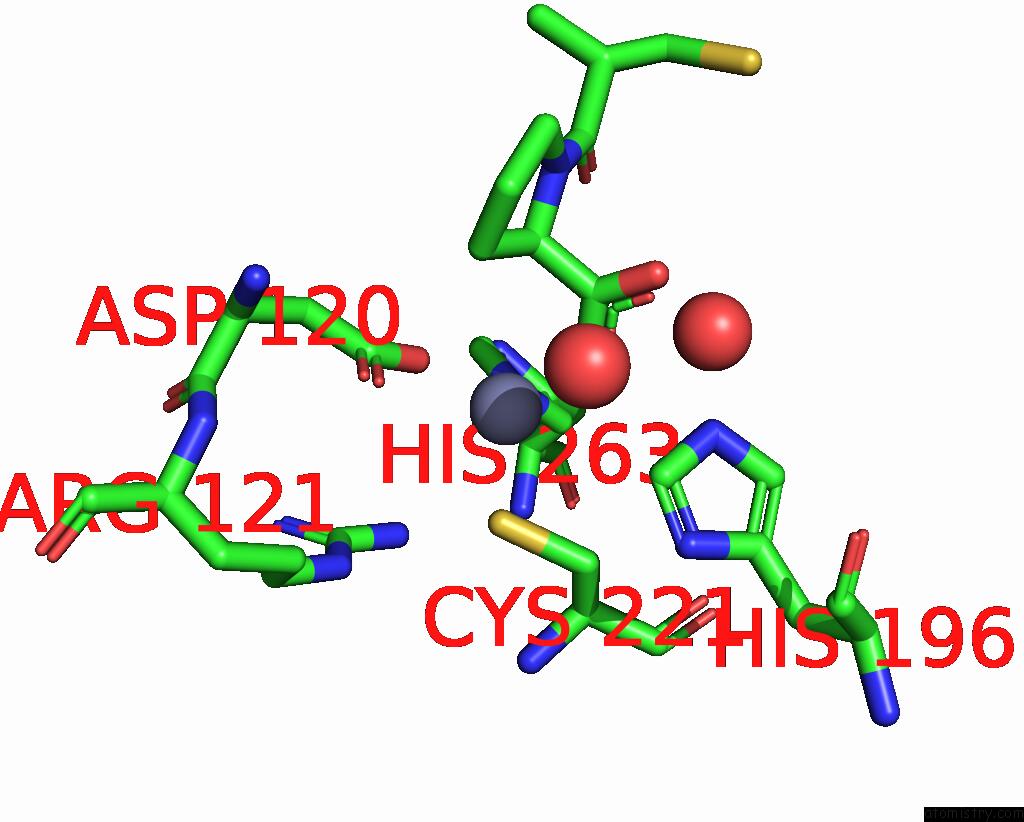
Mono view
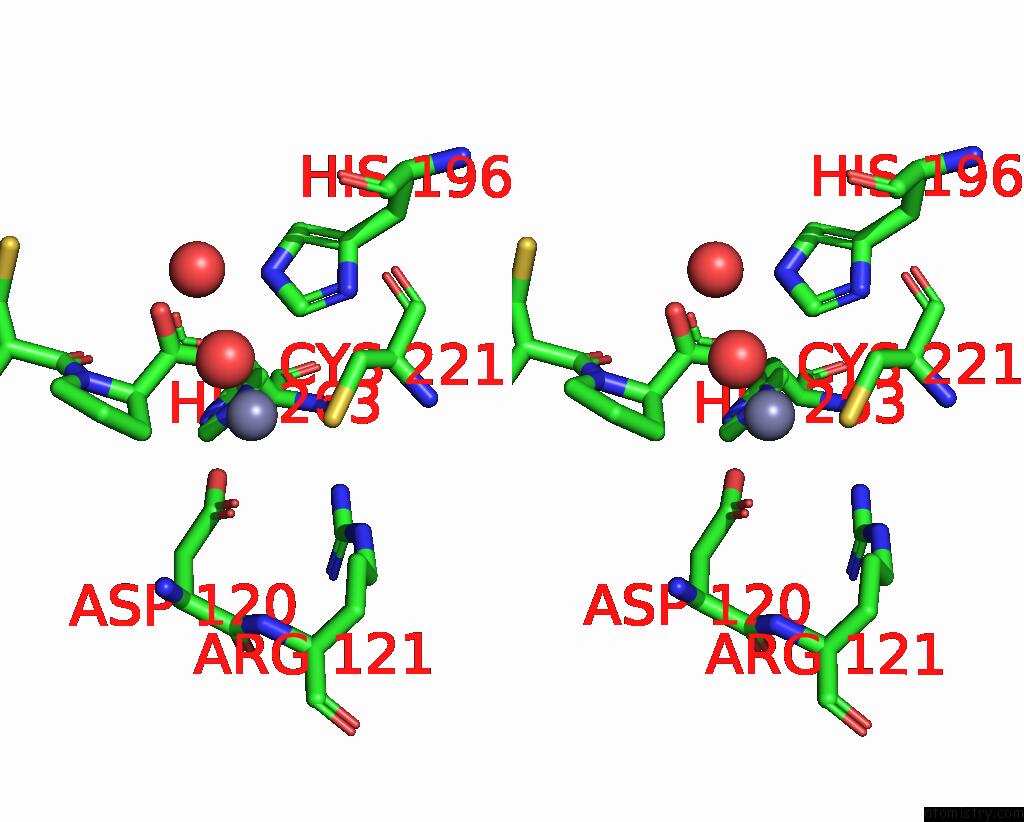
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Crystal Structure of the Zinc Carbapenemase Cpha in Complex with the Inhibitor D-Captopril within 5.0Å range:
|
Reference:
B.M.Lienard,
G.Garau,
L.Horsfall,
A.I.Karsisiotis,
C.Damblon,
P.Lassaux,
C.Papamicael,
G.C.Roberts,
M.Galleni,
O.Dideberg,
J.M.Frere,
C.J.Schofield.
Structural Basis For the Broad-Spectrum Inhibition of Metallo-Beta-Lactamases By Thiols. Org.Biomol.Chem. V. 6 2282 2008.
ISSN: ISSN 1477-0520
PubMed: 18563261
DOI: 10.1039/B802311E
Page generated: Thu Oct 17 03:23:50 2024
ISSN: ISSN 1477-0520
PubMed: 18563261
DOI: 10.1039/B802311E
Last articles
Mg in 3LD0Mg in 3LLU
Mg in 3LK9
Mg in 3LJ0
Mg in 3LDW
Mg in 3LIJ
Mg in 3LIE
Mg in 3LGZ
Mg in 3LHR
Mg in 3LGY