Zinc »
PDB 2naa-2nwy »
2nqz »
Zinc in PDB 2nqz: Trna-Guanine Transglycosylase (Tgt) Mutant in Complex with 7-Deaza-7- Aminomethyl-Guanine
Enzymatic activity of Trna-Guanine Transglycosylase (Tgt) Mutant in Complex with 7-Deaza-7- Aminomethyl-Guanine
All present enzymatic activity of Trna-Guanine Transglycosylase (Tgt) Mutant in Complex with 7-Deaza-7- Aminomethyl-Guanine:
2.4.2.29;
2.4.2.29;
Protein crystallography data
The structure of Trna-Guanine Transglycosylase (Tgt) Mutant in Complex with 7-Deaza-7- Aminomethyl-Guanine, PDB code: 2nqz
was solved by
N.Tidten,
A.Heine,
K.Reuter,
G.Klebe,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 8.00 / 1.46 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 89.900, 64.930, 71.180, 90.00, 96.36, 90.00 |
| R / Rfree (%) | 15 / 19.8 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Trna-Guanine Transglycosylase (Tgt) Mutant in Complex with 7-Deaza-7- Aminomethyl-Guanine
(pdb code 2nqz). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total only one binding site of Zinc was determined in the Trna-Guanine Transglycosylase (Tgt) Mutant in Complex with 7-Deaza-7- Aminomethyl-Guanine, PDB code: 2nqz:
In total only one binding site of Zinc was determined in the Trna-Guanine Transglycosylase (Tgt) Mutant in Complex with 7-Deaza-7- Aminomethyl-Guanine, PDB code: 2nqz:
Zinc binding site 1 out of 1 in 2nqz
Go back to
Zinc binding site 1 out
of 1 in the Trna-Guanine Transglycosylase (Tgt) Mutant in Complex with 7-Deaza-7- Aminomethyl-Guanine
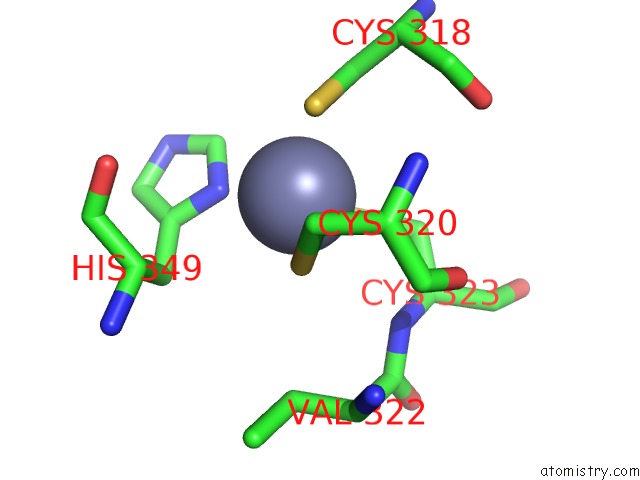
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Trna-Guanine Transglycosylase (Tgt) Mutant in Complex with 7-Deaza-7- Aminomethyl-Guanine within 5.0Å range:
|
Reference:
I.Biela,
N.Tidten-Luksch,
F.Immekus,
S.Glinca,
T.X.Nguyen,
H.D.Gerber,
A.Heine,
G.Klebe,
K.Reuter.
Investigation of Specificity Determinants in Bacterial Trna-Guanine Transglycosylase Reveals Queuine, the Substrate of Its Eucaryotic Counterpart, As Inhibitor Plos One V. 8 64240 2013.
ISSN: ESSN 1932-6203
PubMed: 23704982
DOI: 10.1371/JOURNAL.PONE.0064240
Page generated: Thu Oct 17 02:16:40 2024
ISSN: ESSN 1932-6203
PubMed: 23704982
DOI: 10.1371/JOURNAL.PONE.0064240
Last articles
K in 1QVGK in 1QVF
K in 1RB6
K in 1QY6
K in 1QV0
K in 1QV1
K in 1QU2
K in 1QQN
K in 1QM4
K in 1QQO