Zinc »
PDB 2fac-2foq »
2fea »
Zinc in PDB 2fea: Crystal Structure of Mtnx Phosphatase From Bacillus Subtilis at 2.00 A Resolution
Protein crystallography data
The structure of Crystal Structure of Mtnx Phosphatase From Bacillus Subtilis at 2.00 A Resolution, PDB code: 2fea
was solved by
Joint Center For Structural Genomics (Jcsg),
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 22.95 / 2.00 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 59.013, 43.104, 109.553, 90.00, 98.91, 90.00 |
| R / Rfree (%) | 16 / 21.7 |
Other elements in 2fea:
The structure of Crystal Structure of Mtnx Phosphatase From Bacillus Subtilis at 2.00 A Resolution also contains other interesting chemical elements:
| Magnesium | (Mg) | 2 atoms |
Zinc Binding Sites:
The binding sites of Zinc atom in the Crystal Structure of Mtnx Phosphatase From Bacillus Subtilis at 2.00 A Resolution
(pdb code 2fea). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the Crystal Structure of Mtnx Phosphatase From Bacillus Subtilis at 2.00 A Resolution, PDB code: 2fea:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the Crystal Structure of Mtnx Phosphatase From Bacillus Subtilis at 2.00 A Resolution, PDB code: 2fea:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 2fea
Go back to
Zinc binding site 1 out
of 2 in the Crystal Structure of Mtnx Phosphatase From Bacillus Subtilis at 2.00 A Resolution
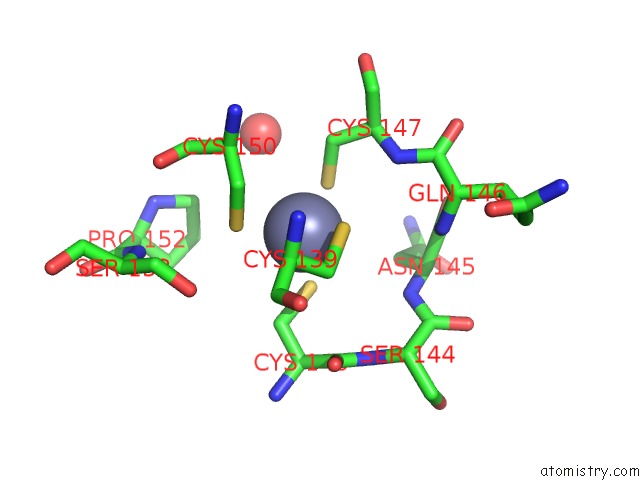
Mono view
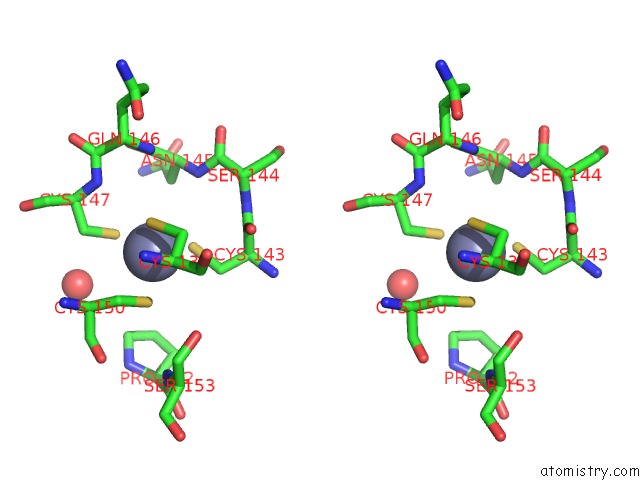
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Crystal Structure of Mtnx Phosphatase From Bacillus Subtilis at 2.00 A Resolution within 5.0Å range:
|
Zinc binding site 2 out of 2 in 2fea
Go back to
Zinc binding site 2 out
of 2 in the Crystal Structure of Mtnx Phosphatase From Bacillus Subtilis at 2.00 A Resolution
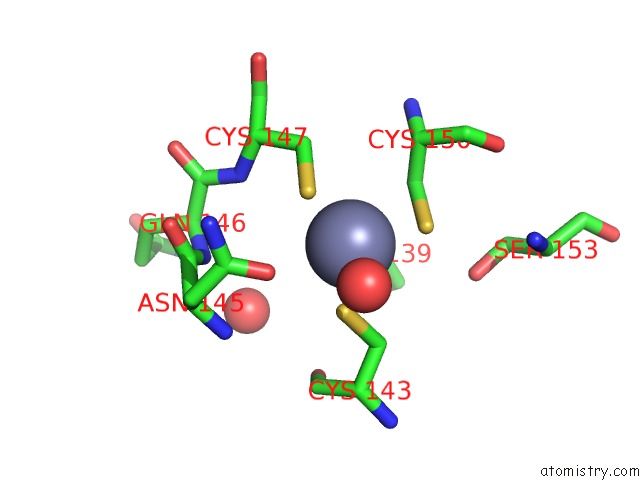
Mono view
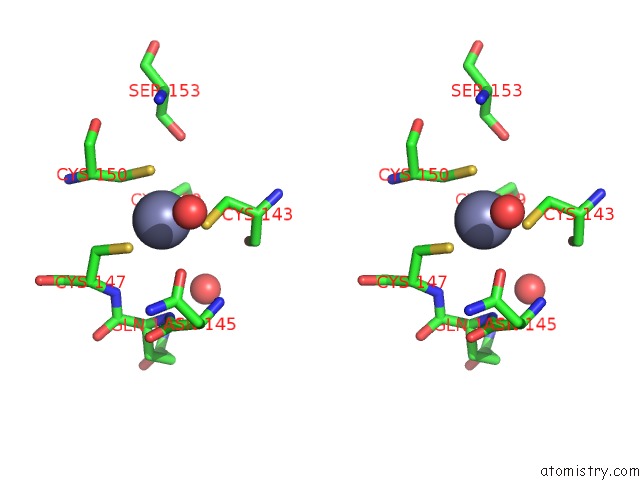
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Crystal Structure of Mtnx Phosphatase From Bacillus Subtilis at 2.00 A Resolution within 5.0Å range:
|
Reference:
Q.Xu,
K.S.Saikatendu,
S.S.Krishna,
D.Mcmullan,
P.Abdubek,
S.Agarwalla,
E.Ambing,
T.Astakhova,
H.L.Axelrod,
D.Carlton,
H.J.Chiu,
T.Clayton,
M.Didonato,
L.Duan,
M.A.Elsliger,
J.Feuerhelm,
S.K.Grzechnik,
J.Hale,
E.Hampton,
G.W.Han,
J.Haugen,
L.Jaroszewski,
K.K.Jin,
H.E.Klock,
M.W.Knuth,
E.Koesema,
M.D.Miller,
A.T.Morse,
E.Nigoghossian,
L.Okach,
S.Oommachen,
J.Paulsen,
R.Reyes,
C.L.Rife,
R.Schwarzenbacher,
H.Van Den Bedem,
A.White,
G.Wolf,
K.O.Hodgson,
J.Wooley,
A.M.Deacon,
A.Godzik,
S.A.Lesley,
I.A.Wilson.
Crystal Structure of Mtnx Phosphatase From Bacillus Subtilis at 2.0 A Resolution Provides A Structural Basis For Bipartite Phosphomonoester Hydrolysis of 2-Hydroxy-3-Keto-5-Methylthiopentenyl-1-Phosphate. Proteins V. 69 433 2007.
ISSN: ISSN 0887-3585
PubMed: 17654724
DOI: 10.1002/PROT.21602
Page generated: Wed Oct 16 23:45:06 2024
ISSN: ISSN 0887-3585
PubMed: 17654724
DOI: 10.1002/PROT.21602
Last articles
Mg in 4QR8Mg in 4QRD
Mg in 4QQF
Mg in 4QR9
Mg in 4QQ8
Mg in 4QQO
Mg in 4QPZ
Mg in 4QPM
Mg in 4QPF
Mg in 4QPJ