Zinc »
PDB 1zsc-2a2i »
1zx1 »
Zinc in PDB 1zx1: Human Quinone Oxidoreductase 2 (NQO2) in Complex with the Cytostatic Prodrug CB1954
Protein crystallography data
The structure of Human Quinone Oxidoreductase 2 (NQO2) in Complex with the Cytostatic Prodrug CB1954, PDB code: 1zx1
was solved by
A.Jansson,
X.Wu,
K.Kavanagh,
D.Kerr,
R.Knox,
R.Walton,
U.Gunther,
C.Ludwig,
A.Edwards,
C.Arrowsmith,
M.Sundstrom,
F.Von Delft,
U.Oppermann,
Structural Genomics Consortium (Sgc),
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 50.00 / 2.16 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 56.328, 84.341, 106.579, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17.4 / 20.7 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Human Quinone Oxidoreductase 2 (NQO2) in Complex with the Cytostatic Prodrug CB1954
(pdb code 1zx1). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the Human Quinone Oxidoreductase 2 (NQO2) in Complex with the Cytostatic Prodrug CB1954, PDB code: 1zx1:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the Human Quinone Oxidoreductase 2 (NQO2) in Complex with the Cytostatic Prodrug CB1954, PDB code: 1zx1:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 1zx1
Go back to
Zinc binding site 1 out
of 2 in the Human Quinone Oxidoreductase 2 (NQO2) in Complex with the Cytostatic Prodrug CB1954
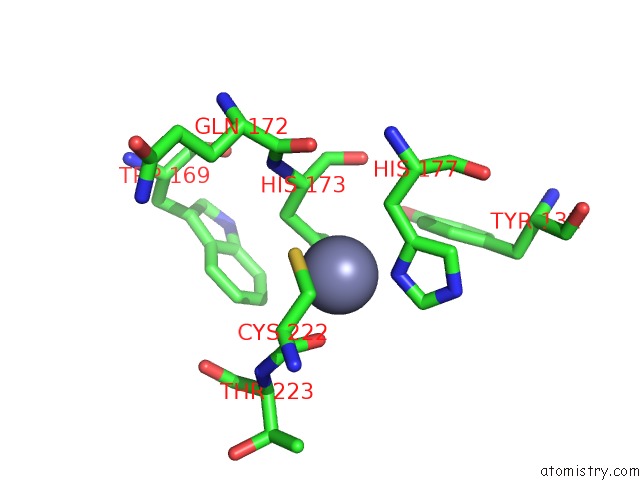
Mono view
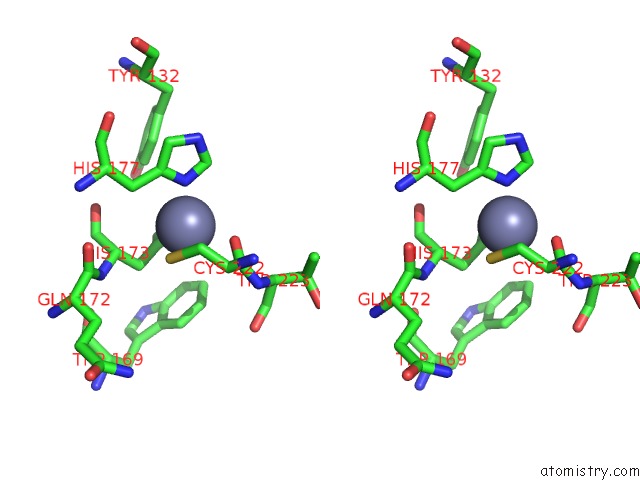
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Human Quinone Oxidoreductase 2 (NQO2) in Complex with the Cytostatic Prodrug CB1954 within 5.0Å range:
|
Zinc binding site 2 out of 2 in 1zx1
Go back to
Zinc binding site 2 out
of 2 in the Human Quinone Oxidoreductase 2 (NQO2) in Complex with the Cytostatic Prodrug CB1954
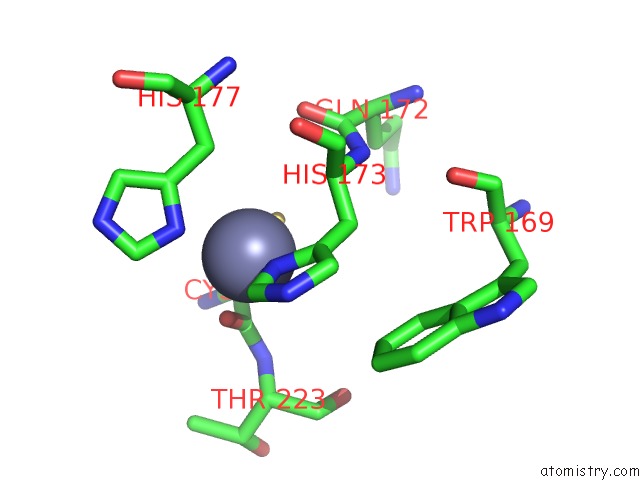
Mono view
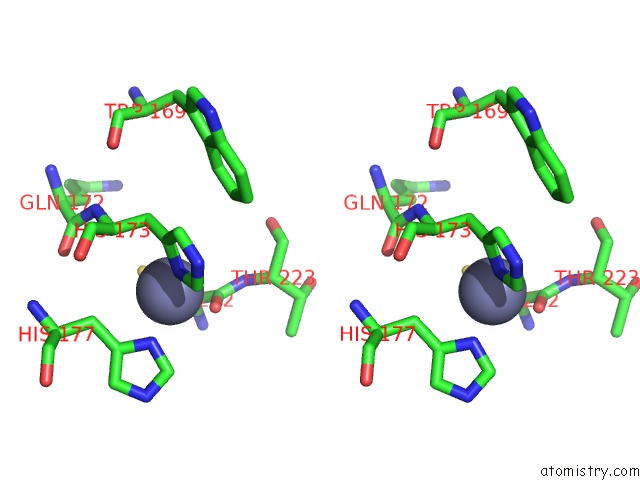
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Human Quinone Oxidoreductase 2 (NQO2) in Complex with the Cytostatic Prodrug CB1954 within 5.0Å range:
|
Reference:
A.Jansson,
X.Wu,
K.Kavanagh,
D.Kerr,
R.Knox,
R.Walton,
U.Gunther,
C.Ludwig,
A.Edwards,
C.Arrowsmith,
M.Sundstrom,
F.Von Delft,
U.Oppermann.
Human Quinone Oxidoreductase 2 (NQO2) in Complex with the Cytostatic Prodrug CB1954 To Be Published.
Page generated: Wed Aug 20 01:00:03 2025
Last articles
Zn in 2N6JZn in 2N26
Zn in 2N5K
Zn in 2N1A
Zn in 2N25
Zn in 2N1U
Zn in 2MZZ
Zn in 2MZI
Zn in 2MZH
Zn in 2MY1