Zinc »
PDB 1tbt-1to4 »
1tnb »
Zinc in PDB 1tnb: Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21
Enzymatic activity of Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21
All present enzymatic activity of Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21:
2.5.1.59;
2.5.1.59;
Protein crystallography data
The structure of Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21, PDB code: 1tnb
was solved by
T.S.Reid,
K.L.Terry,
P.J.Casey,
L.S.Beese,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 27.66 / 2.85 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 271.130, 266.720, 185.141, 90.00, 131.68, 90.00 |
| R / Rfree (%) | 18.9 / 21 |
Other elements in 1tnb:
The structure of Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21 also contains other interesting chemical elements:
| Chlorine | (Cl) | 3 atoms |
Zinc Binding Sites:
The binding sites of Zinc atom in the Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21
(pdb code 1tnb). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 6 binding sites of Zinc where determined in the Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21, PDB code: 1tnb:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Zinc where determined in the Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21, PDB code: 1tnb:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6;
Zinc binding site 1 out of 6 in 1tnb
Go back to
Zinc binding site 1 out
of 6 in the Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21
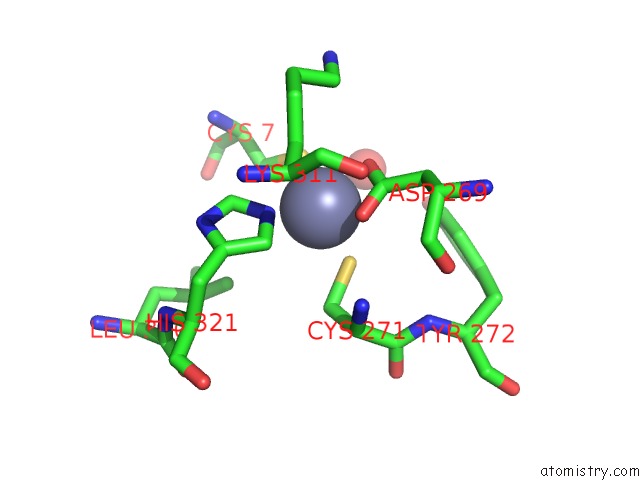
Mono view
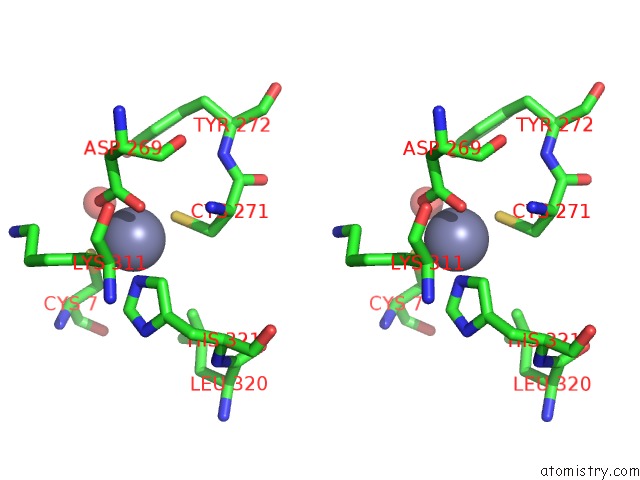
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21 within 5.0Å range:
|
Zinc binding site 2 out of 6 in 1tnb
Go back to
Zinc binding site 2 out
of 6 in the Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21
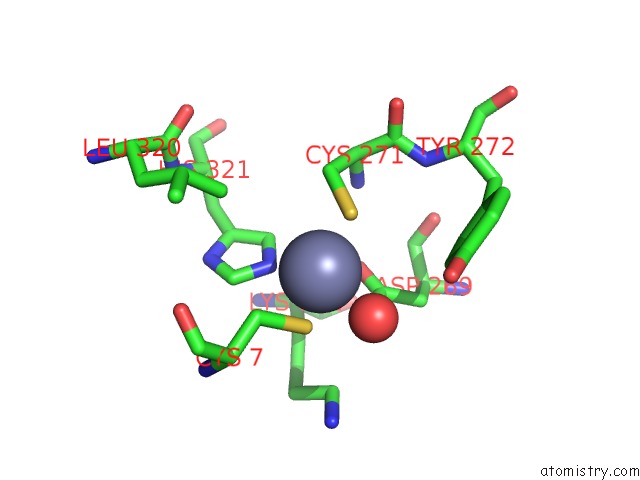
Mono view
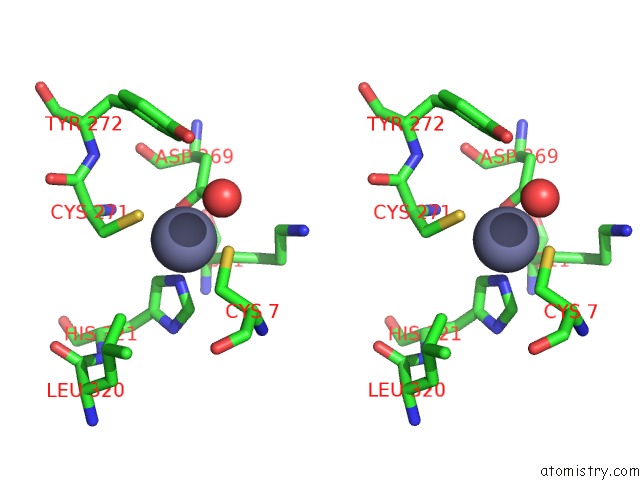
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21 within 5.0Å range:
|
Zinc binding site 3 out of 6 in 1tnb
Go back to
Zinc binding site 3 out
of 6 in the Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21
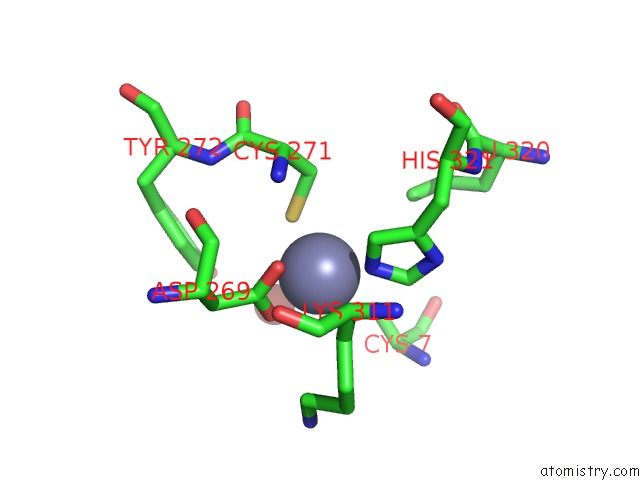
Mono view
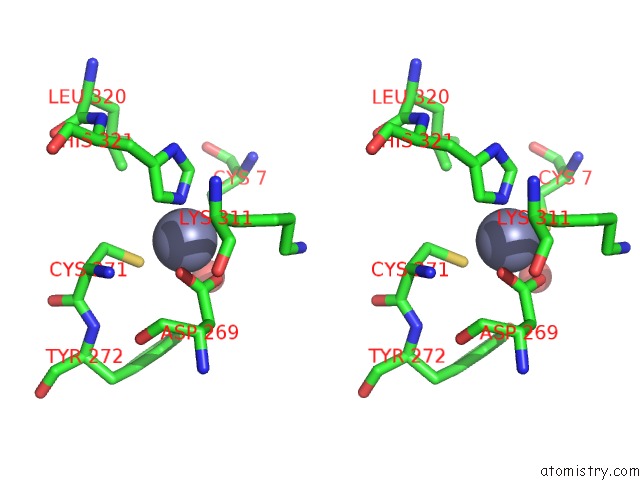
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21 within 5.0Å range:
|
Zinc binding site 4 out of 6 in 1tnb
Go back to
Zinc binding site 4 out
of 6 in the Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21
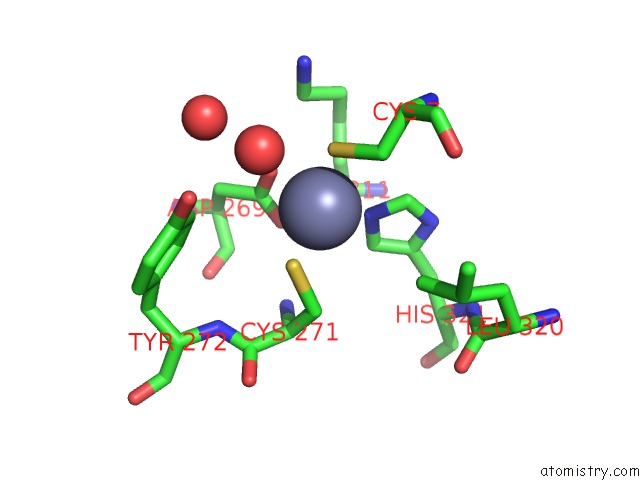
Mono view
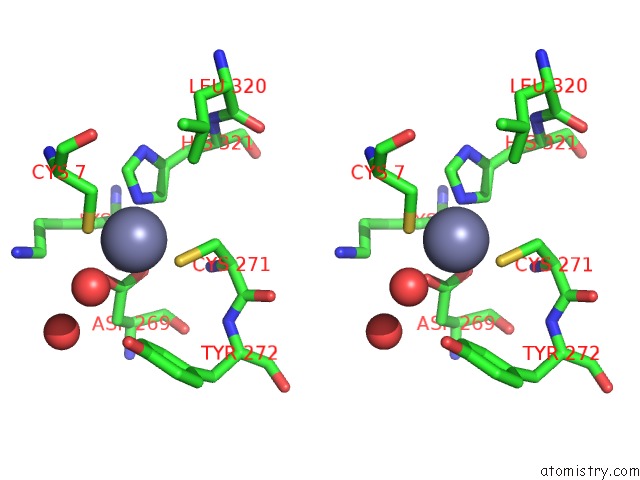
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21 within 5.0Å range:
|
Zinc binding site 5 out of 6 in 1tnb
Go back to
Zinc binding site 5 out
of 6 in the Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21
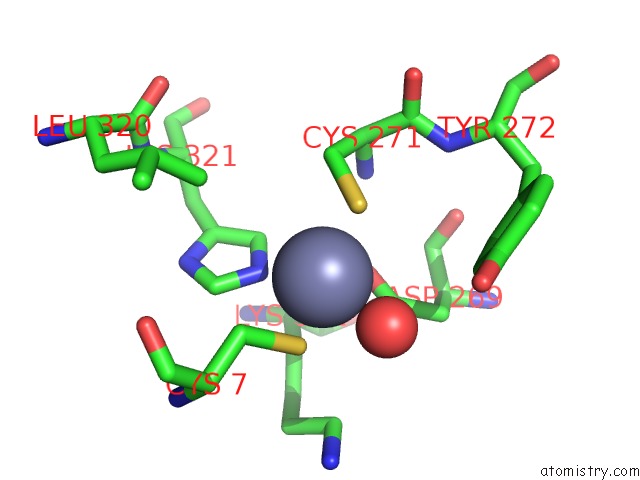
Mono view
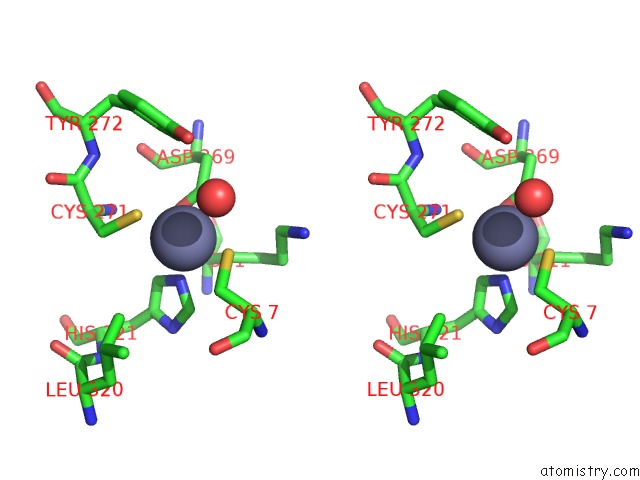
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 5 of Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21 within 5.0Å range:
|
Zinc binding site 6 out of 6 in 1tnb
Go back to
Zinc binding site 6 out
of 6 in the Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21
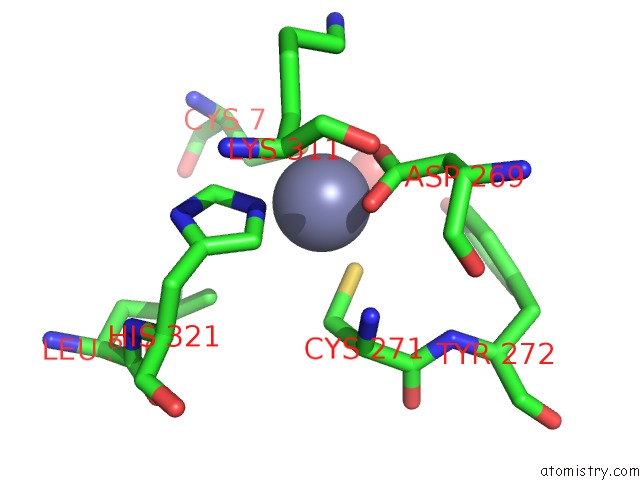
Mono view
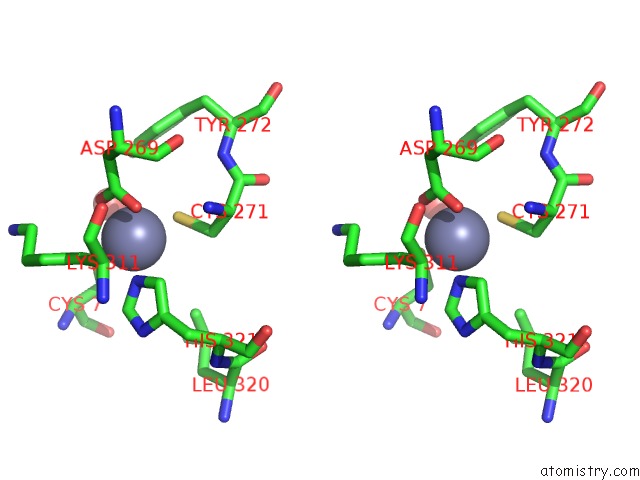
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 6 of Rat Protein Geranylgeranyltransferase Type-I Complexed with A Ggpp Analog and A Substrate Kksktkcvif Peptide Derived From TC21 within 5.0Å range:
|
Reference:
T.S.Reid,
K.L.Terry,
P.J.Casey,
L.S.Beese.
Crystallographic Analysis of Caax Prenyltransferases Complexed with Substrates Defines Rules of Protein Substrate Selectivity. J.Mol.Biol. V. 343 417 2004.
ISSN: ISSN 0022-2836
PubMed: 15451670
DOI: 10.1016/J.JMB.2004.08.056
Page generated: Wed Oct 16 19:12:10 2024
ISSN: ISSN 0022-2836
PubMed: 15451670
DOI: 10.1016/J.JMB.2004.08.056
Last articles
K in 7QR0K in 7QR1
K in 7QQZ
K in 7QQX
K in 7QQW
K in 7QQU
K in 7QQV
K in 7QQT
K in 7QQR
K in 7QQS