Zinc »
PDB 1h71-1hld »
1hbu »
Zinc in PDB 1hbu: Methyl-Coenzyme M Reductase in the Mcr-RED1-Silent State in Complex with Coenzyme M
Protein crystallography data
The structure of Methyl-Coenzyme M Reductase in the Mcr-RED1-Silent State in Complex with Coenzyme M, PDB code: 1hbu
was solved by
U.Ermler,
W.Grabarse,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 10.00 / 1.90 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 81.100, 116.500, 121.800, 90.00, 91.80, 90.00 |
| R / Rfree (%) | 16.2 / 21.1 |
Other elements in 1hbu:
The structure of Methyl-Coenzyme M Reductase in the Mcr-RED1-Silent State in Complex with Coenzyme M also contains other interesting chemical elements:
| Nickel | (Ni) | 2 atoms |
| Magnesium | (Mg) | 13 atoms |
| Chlorine | (Cl) | 2 atoms |
| Sodium | (Na) | 9 atoms |
Zinc Binding Sites:
The binding sites of Zinc atom in the Methyl-Coenzyme M Reductase in the Mcr-RED1-Silent State in Complex with Coenzyme M
(pdb code 1hbu). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total only one binding site of Zinc was determined in the Methyl-Coenzyme M Reductase in the Mcr-RED1-Silent State in Complex with Coenzyme M, PDB code: 1hbu:
In total only one binding site of Zinc was determined in the Methyl-Coenzyme M Reductase in the Mcr-RED1-Silent State in Complex with Coenzyme M, PDB code: 1hbu:
Zinc binding site 1 out of 1 in 1hbu
Go back to
Zinc binding site 1 out
of 1 in the Methyl-Coenzyme M Reductase in the Mcr-RED1-Silent State in Complex with Coenzyme M
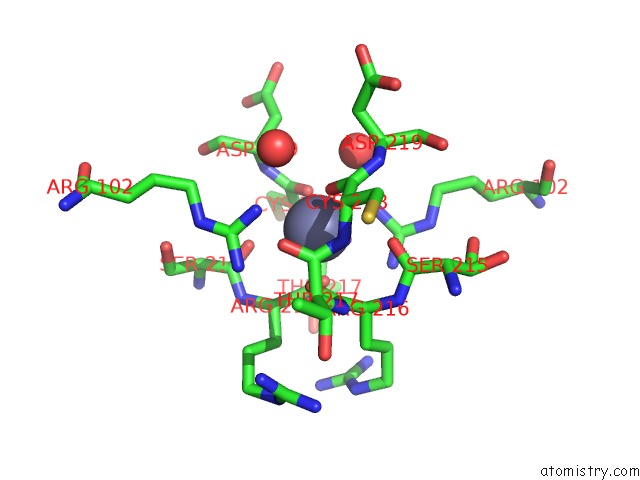
Mono view
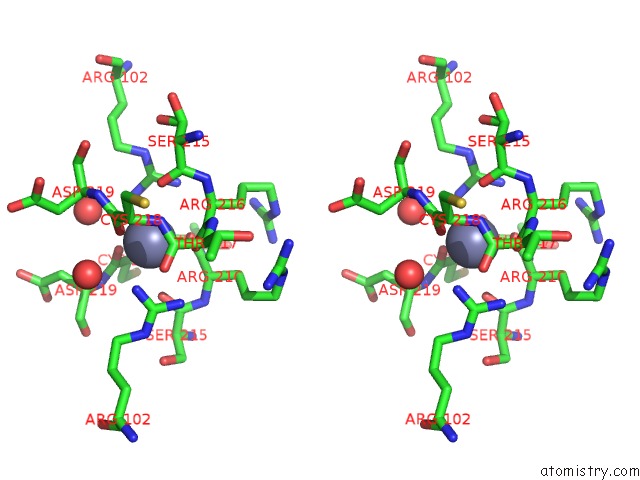
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Methyl-Coenzyme M Reductase in the Mcr-RED1-Silent State in Complex with Coenzyme M within 5.0Å range:
|
Reference:
W.Grabarse,
F.Mahlert,
E.C.Duin,
M.Goubeaud,
S.Shima,
R.K.Thauer,
V.Lamzin,
U.Ermler.
On the Mechanism of Biological Methane Formation: Structural Evidence For Conformational Changes in Methyl-Coenzyme M Reductase Upon Substrate Binding J.Mol.Biol. V. 309 315 2001.
ISSN: ISSN 0022-2836
PubMed: 11491299
DOI: 10.1006/JMBI.2001.4647
Page generated: Sun Oct 13 02:05:55 2024
ISSN: ISSN 0022-2836
PubMed: 11491299
DOI: 10.1006/JMBI.2001.4647
Last articles
Fe in 3MINFe in 3MK7
Fe in 3MM3
Fe in 3MM2
Fe in 3MM1
Fe in 3ML1
Fe in 3MKB
Fe in 3MJP
Fe in 3MJU
Fe in 3MI5