Zinc in PDB 1z3j: Solution Structure of MMP12 in the Presence of N-Isobutyl-N- 4-Methoxyphenylsulfonyl]Glycyl Hydroxamic Acid (Nngh)
Enzymatic activity of Solution Structure of MMP12 in the Presence of N-Isobutyl-N- 4-Methoxyphenylsulfonyl]Glycyl Hydroxamic Acid (Nngh)
All present enzymatic activity of Solution Structure of MMP12 in the Presence of N-Isobutyl-N- 4-Methoxyphenylsulfonyl]Glycyl Hydroxamic Acid (Nngh):
3.4.24.65;
3.4.24.65;
Other elements in 1z3j:
The structure of Solution Structure of MMP12 in the Presence of N-Isobutyl-N- 4-Methoxyphenylsulfonyl]Glycyl Hydroxamic Acid (Nngh) also contains other interesting chemical elements:
| Calcium | (Ca) | 3 atoms |
Zinc Binding Sites:
The binding sites of Zinc atom in the Solution Structure of MMP12 in the Presence of N-Isobutyl-N- 4-Methoxyphenylsulfonyl]Glycyl Hydroxamic Acid (Nngh)
(pdb code 1z3j). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the Solution Structure of MMP12 in the Presence of N-Isobutyl-N- 4-Methoxyphenylsulfonyl]Glycyl Hydroxamic Acid (Nngh), PDB code: 1z3j:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the Solution Structure of MMP12 in the Presence of N-Isobutyl-N- 4-Methoxyphenylsulfonyl]Glycyl Hydroxamic Acid (Nngh), PDB code: 1z3j:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 1z3j
Go back to
Zinc binding site 1 out
of 2 in the Solution Structure of MMP12 in the Presence of N-Isobutyl-N- 4-Methoxyphenylsulfonyl]Glycyl Hydroxamic Acid (Nngh)
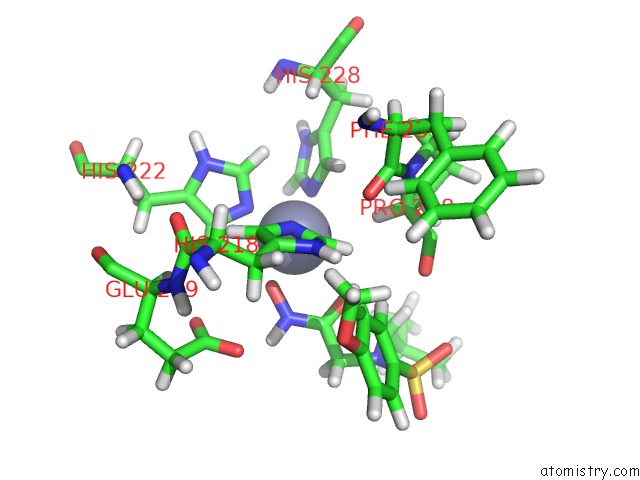
Mono view
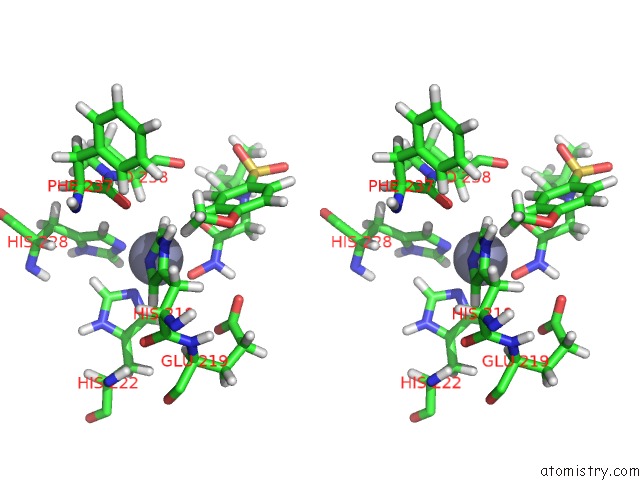
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Solution Structure of MMP12 in the Presence of N-Isobutyl-N- 4-Methoxyphenylsulfonyl]Glycyl Hydroxamic Acid (Nngh) within 5.0Å range:
|
Zinc binding site 2 out of 2 in 1z3j
Go back to
Zinc binding site 2 out
of 2 in the Solution Structure of MMP12 in the Presence of N-Isobutyl-N- 4-Methoxyphenylsulfonyl]Glycyl Hydroxamic Acid (Nngh)
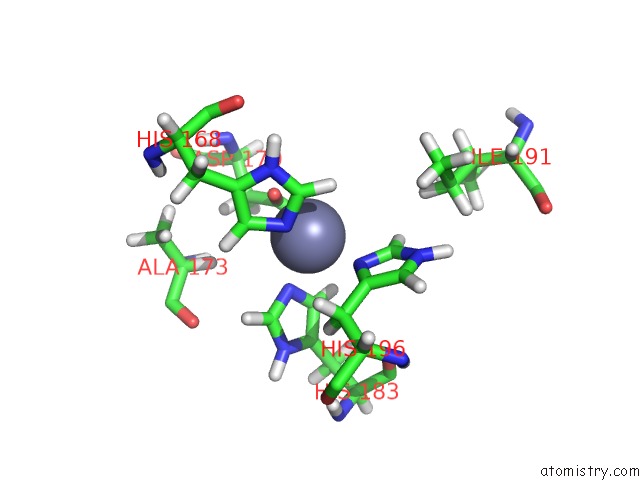
Mono view
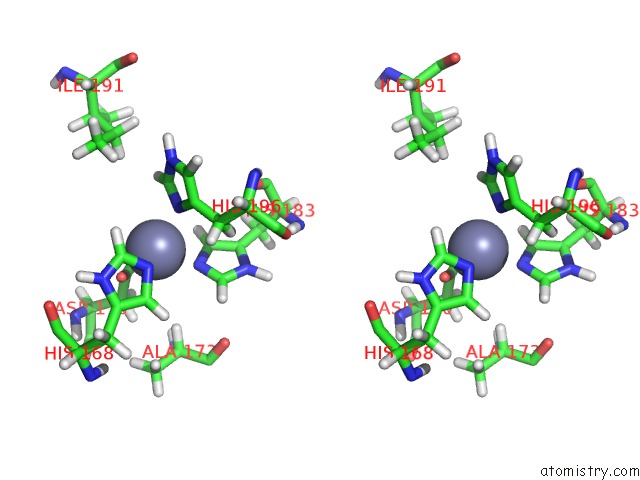
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Solution Structure of MMP12 in the Presence of N-Isobutyl-N- 4-Methoxyphenylsulfonyl]Glycyl Hydroxamic Acid (Nngh) within 5.0Å range:
|
Reference:
I.Bertini,
V.Calderone,
M.Cosenza,
M.Fragai,
Y.M.Lee,
C.Luchinat,
S.Mangani,
B.Terni,
P.Turano.
Conformational Variability of Matrix Metalloproteinases: Beyond A Single 3D Structure. Proc.Natl.Acad.Sci.Usa V. 102 5334 2005.
ISSN: ISSN 0027-8424
PubMed: 15809432
DOI: 10.1073/PNAS.0407106102
Page generated: Wed Oct 16 21:06:57 2024
ISSN: ISSN 0027-8424
PubMed: 15809432
DOI: 10.1073/PNAS.0407106102
Last articles
Zn in 9J0NZn in 9J0O
Zn in 9J0P
Zn in 9FJX
Zn in 9EKB
Zn in 9C0F
Zn in 9CAH
Zn in 9CH0
Zn in 9CH3
Zn in 9CH1